Asia Pac Allergy.
2015 Jul;5(3):145-155. 10.5415/apallergy.2015.5.3.145.
Association of prevalence of rhinitis, atopic eczema, rhinoconjunctivitis and wheezing with mortality from infectious diseases and with antibiotic susceptibility at a country level
- Affiliations
-
- 1Department of Medicine, Mater Dei Hospital, Msida, MSD 2090, Malta. claudiafsadni@gmail.com
- 2Faculty of Medicine and Surgery, University of Malta, Msida, MSD 2090, Malta.
- KMID: 2397044
- DOI: http://doi.org/10.5415/apallergy.2015.5.3.145
Abstract
- BACKGROUND
It was previously reported that there is a positive correlation between incidence of type 1 diabetes and prevalence of asthma and atopic eczema. A negative correlation between the prevalence of type 1 diabetes and mortality from infectious diseases as well as a positive correlation with antibiotic susceptibility at a country level have also been reported.
OBJECTIVE
The aim of this study was to investigate the association between country prevalence of rhinitis, atopic eczema, rhinoconjunctivitis, and wheezing with mortality from infectious diseases and also with antibiotic susceptibility at a country level.
METHODS
Data for prevalence of rhinitis, eczema, rhinoconjunctivitis, and wheezing was obtained from the International Study of Asthma and Allergies in Childhood study (ISAAC). ISAAC Phase one was a multicentre multicountry cross sectional study involving over 700,000 children in 2 age groups of school children, 13-14 years old (adolescents) and 6-7 years old (children) in 156 centres from 56 countries. Mortality from infectious diseases was taken from World Health Organisation data. The Alexander project was used to identify antibiotic susceptibilities to common bacteria.
RESULTS
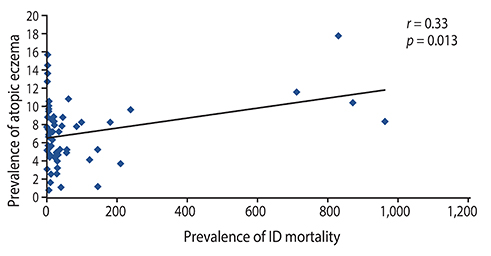
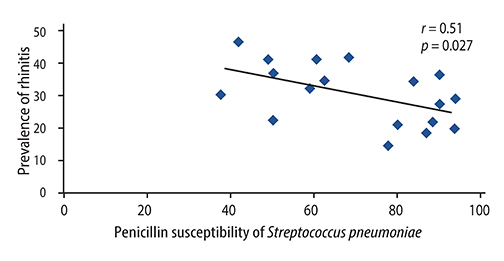
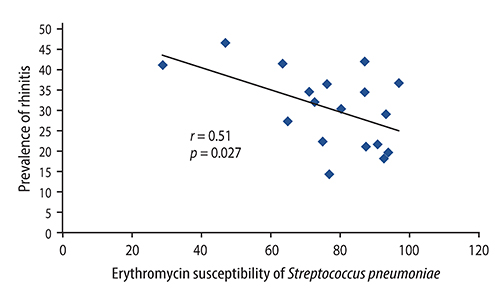
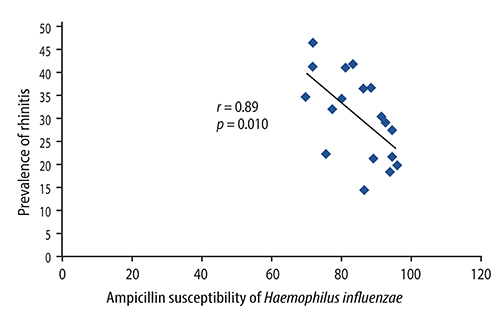
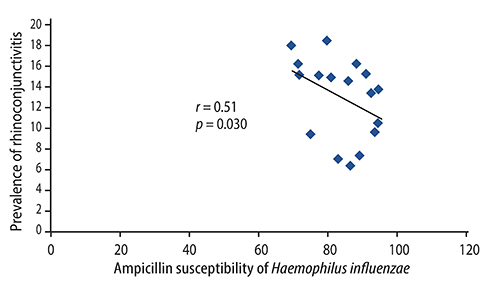
There were significant positive correlations between atopic eczema and mortality from all infectious diseases studied, diarrhoeal illness, tropical infections, and childhood infections. A negative correlation exists between the prevalence of rhinitis and Streptococcus pneumoniae susceptibility to penicillin and to erythromycin, rhinitis and Haemophilus influenzae susceptibility to ampicillin and between rhinoconjunctivitis and H. influenzae susceptibility to ampicillin.
CONCLUSION
Th1/Th2 responses might influence the pathogenesis of infectious disease mortality, while antibiotic overprescription could explain the negative association between atopy and antibiotic susceptibility.
Keyword
MeSH Terms
-
Ampicillin
Anti-Infective Agents
Asthma
Bacteria
Child
Communicable Diseases*
Conjunctivitis, Allergic
Dermatitis, Atopic*
Eczema
Erythromycin
Global Health
Haemophilus influenzae
Humans
Hypersensitivity
Incidence
Influenza, Human
Mortality*
Penicillins
Prevalence*
Respiratory Sounds*
Rhinitis*
Streptococcus pneumoniae
Ampicillin
Anti-Infective Agents
Erythromycin
Penicillins
Figure
Cited by 1 articles
-
The sticky relationship between allergies and infections
Jiu-Yao Wang
Asia Pac Allergy. 2015;5(3):133-135. doi: 10.5415/apallergy.2015.5.3.133.
Reference
-
1. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003; 112:6 Suppl. S118–S127.
Article2. Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Bjorksten B, Burr M, Clayton T, Crane J, Ellwood P, Keil U, Lai C, Mallol J, Martinez F, Mitchell E, Montefort S, Pearce N, Shah J, Sibbald B, Strachan D, von Mutius E, Weiland SK. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999; 103(1 Pt 1):125–138.
Article3. Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011; 131:67–73.
Article4. Trepka MJ, Heinrich J, Wichmann HE. The epidemiology of atopic diseases in Germany: an east-west comparison. Rev Environ Health. 1996; 11:119–131.
Article5. Fsadni P, Fsadni C, Fava S, Montefort S. Correlation of worldwide incidence of type 1 diabetes (DiaMond) with prevalence of asthma and atopic eczema (ISAAC). Clin Respir J. 2012; 6:18–25.
Article6. Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002; 45:588–594.
Article7. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000; 55:Suppl 1. S2–S10.
Article8. Abela AG, Fava S. Association of incidence of type 1 diabetes with mortality from infectious disease and with antibiotic susceptibility at a country level. Acta Diabetol. 2013; 50:859–865.
Article9. International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee [Internet]. Auckland (NZ): ISAAC;2010. cited 2014 Jan 30. Available from: http://isaac.auckland.ac.nz.10. Mathers CD, Bernard C, Iburg K, Inoue M, Ma Fat D, Shibuya K, Stein C, Tomijima N, Xu H. Global burden of disease in 2002: data sources, methods and results. Global programme on evidence for health policy discussion paper No. 54 [Internet]. Geneva: World Health Organization;c2015. cited 2014 Jan 30. Available from: http://www.who.int/healthinfo/paper54.pdf.11. World Health Organization. The World Health Report 2004: changing history [Internet]. Geneva: World Health Organization;c2015. cited 2014 Jan 30. Available from: http://www.who.int/whr/2004/en/.12. Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN. Alexander Project Group. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003; 52:229–246.
Article13. Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009; 129:543–552.
Article14. Akdis M, Trautmann A, Klunker S, Daigle I, Kucuksezer UC, Deglmann W, Disch R, Blaser K, Akdis CA. T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003; 17:1026–1035.
Article15. Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008; 121:652–658.e1.16. Ehrchen JM, Roth J, Roebrock K, Varga G, Domschke W, Newberry R, Sorg C, Muller-Tidow C, Sunderkotter C, Kucharzik T, Spahn TW. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect Immun. 2008; 76:4241–4250.17. Fernandez-Cabezudo MJ, Ali SA, Ullah A, Hasan MY, Kosanovic M, Fahim MA, Adem A, al-Ramadi BK. Pronounced susceptibility to infection by Salmonella enterica serovar Typhimurium in mice chronically exposed to lead correlates with a shift to Th2-type immune responses. Toxicol Appl Pharmacol. 2007; 218:215–226.
Article18. Arendse B, Van Snick J, Brombacher F. IL-9 is a susceptibility factor in Leishmania major infection by promoting detrimental Th2/type 2 responses. J Immunol. 2005; 174:2205–2211.19. Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, Stanciu LA, Gnesini G, Pastore A, Spanevello A, Morelli P, Johnston SL, Caramori G, Papi A. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015; 04. 08. [Epub]. DOI: 10.1111/all.12627.
Article20. Bhattacharya D, Dwivedi VP, Kumar S, Reddy MC, Van Kaer L, Moodley P, Das G. Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances Bacillus Calmette-Guerin vaccine efficacy against tuberculosis. J Biol Chem. 2014; 289:33404–33411.
Article21. Schmitz R, Atzpodien K, Schlaud M. Prevalence and risk factors of atopic diseases in German children and adolescents. Pediatr Allergy Immunol. 2012; 23:716–723.
Article22. Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol. 2012; 46:Suppl. S56–S63.
Article23. Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, Clerici M, Ricci E, Fusi A, De Vecchi E, Piconi S, Drago L. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012; 46:Suppl. S33–S40.
Article24. Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, Macpherson AJ, Strugnell R, von Mering C, Hardt WD. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010; 6:e1001097.
Article25. Mrabet-Dahbi S, Maurer M. Does allergy impair innate immunity? Leads and lessons from atopic dermatitis. Allergy. 2010; 65:1351–1356.
Article26. Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009; 129:1878–1891.
Article27. Simon D, Kozlowski E, Simon H. Natural killer T cells expressing IFN-gamma and IL-4 in lesional skin of atopic eczema. Allergy. 2009; 64:1681–1684.28. Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992; 90:1479–1485.
Article29. Gros E, Petzold S, Maintz L, Bieber T, Novak N. Reduced IFN-γ receptor expression and attenuated IFN-γ response by dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2011; 128:1015–1021.
Article30. Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000; 164:6406–6416.
Article31. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014; 133:1041–1047.32. Ciprandi G, Tosca MA, Fasce L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr Allergy Immunol. 2006; 17:389–391.
Article33. Cirillo I, Marseglia G, Klersy C, Ciprandi G. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy. 2007; 62:1087–1090.
Article34. Ardern-Jones MR, Black AP, Bateman EA, Ogg GS. Bacterial superantigen facilitates epithelial presentation of allergen to T helper 2 cells. Proc Natl Acad Sci U S A. 2007; 104:5557–5562.
Article35. Hussain I, Smith J. Evidence for the transmissibility of atopy: hypothesis. Chest. 2003; 124:1968–1974.36. Baboonian C, Venables PJ, Williams DG, Williams RO, Maini RN. Cross reaction of antibodies to a glycine/alanine repeat sequence of Epstein-Barr virus nuclear antigen-1 with collagen, cytokeratin, and actin. Ann Rheum Dis. 1991; 50:772–775.
Article37. Lappalainen MH, Hyvarinen A, Hirvonen MR, Rintala H, Roivainen J, Renz H, Pfefferle PI, Nevalainen A, Roponen M, Pekkanen J. High indoor microbial levels are associated with reduced Th1 cytokine secretion capacity in infancy. Int Arch Allergy Immunol. 2012; 159:194–203.
Article38. Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, Shirakawa T, Sonomoto K, Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009; 56:80–87.
Article39. Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, Newburg DS, Ward DV, Schibler KR. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014; 165:23–29.
Article40. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012; 129:434–440. 440.e1–440.e2.
Article41. Forno E, Onderdonk AB, McCracken J, Litonjua AA, Laskey D, Delaney ML, Dubois AM, Gold DR, Ryan LM, Weiss ST, Celedon JC. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008; 6:11.
Article42. Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007; 56:661–667.
Article43. Salava A, Lauerma A. Role of the skin microbiome in atopic dermatitis. Clin Transl Allergy. 2014; 4:33.
Article44. Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, Robertson C. ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007; 62:758–766.
Article45. Asher MI, Stewart AW, Wong G, Strachan DP, Garcia-Marcos L, Anderson HR. ISAAC Phase Three Study Group. Changes over time in the relationship between symptoms of asthma, rhinoconjunctivitis and eczema: a global perspective from the International Study of Asthma and Allergies in Childhood (ISAAC). Allergol Immunopathol (Madr). 2012; 40:267–274.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Atopic Dermatitis, Wheezing during Infancy and Asthma Development
- Prevalence of Asthma, Rhinitis and Eczema in Korean Children Using the International Study of Asthma and Allergies in Childhood (ISAAC) Questionnaires
- Dermographism ( IV ): The Prevalence in Atopic Dermatitis and Allergic Rhinitis
- The Burden of Rhinitis and Rhinoconjunctivitis in Adolescents
- Distinct effect of sensitization of house dust mite and citrus red mite (Panonychus citri) in the development of allergic diseases in 16-18 year old adolescents living in rural areas of Jeju island