Korean J Physiol Pharmacol.
2017 Nov;21(6):675-686. 10.4196/kjpp.2017.21.6.675.
Dual control of the vestibulosympathetic reflex following hypotension in rats
- Affiliations
-
- 1Department of Orthopedic Surgery, Kyung Hee University Hospital, Seoul 02447, Korea.
- 2Department of Physiology and Pathophysiology, Yanbian University College of Medicine, Yanji 133002, China.
- 3Department of Physiology, Wonkwang University of School of Medicine and Brain Science Institute at Wonkwang University, Iksan 54538, Korea. byungp@wku.ac.kr
- KMID: 2395263
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.675
Abstract
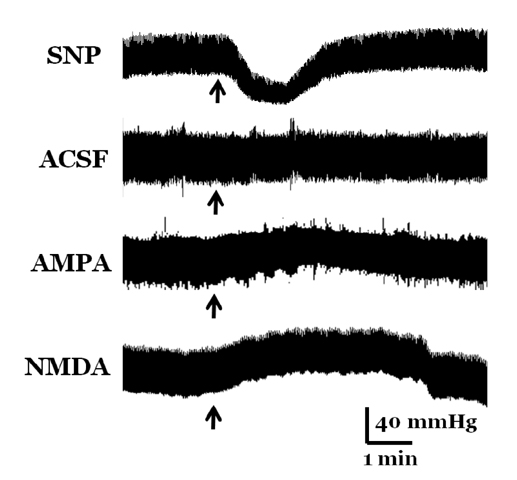
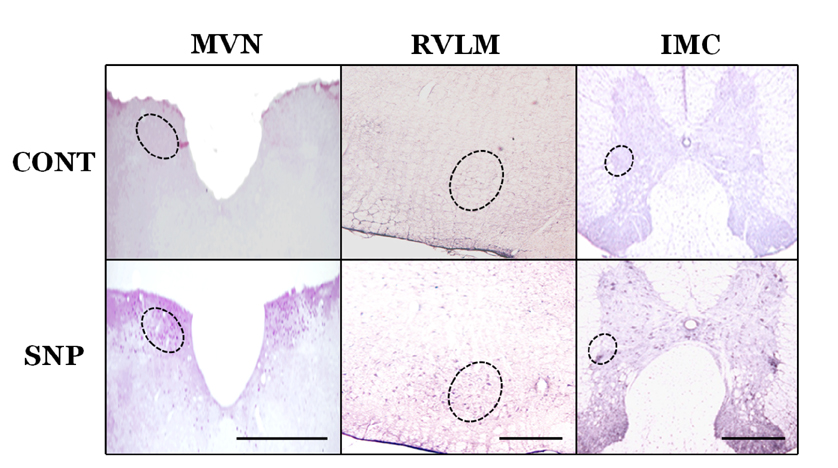
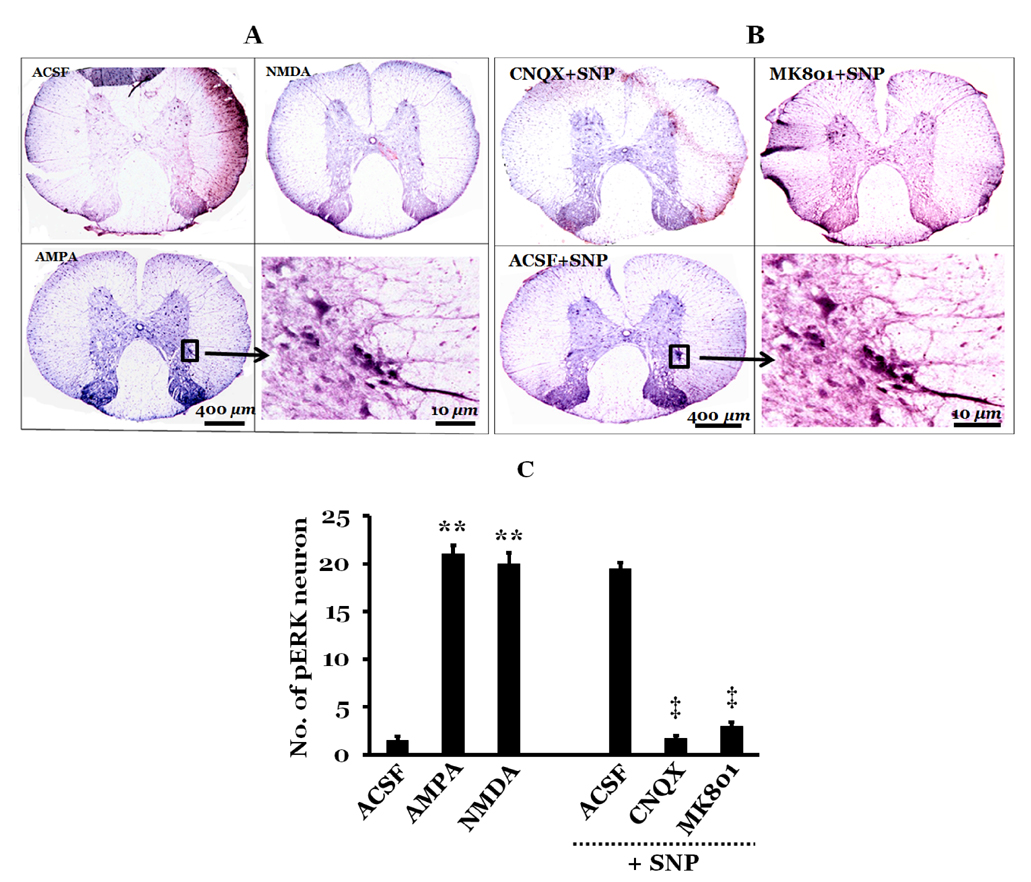
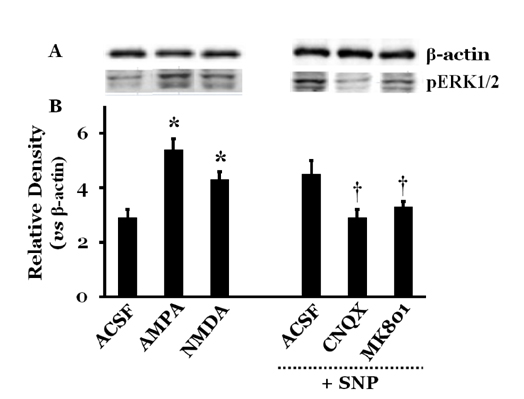
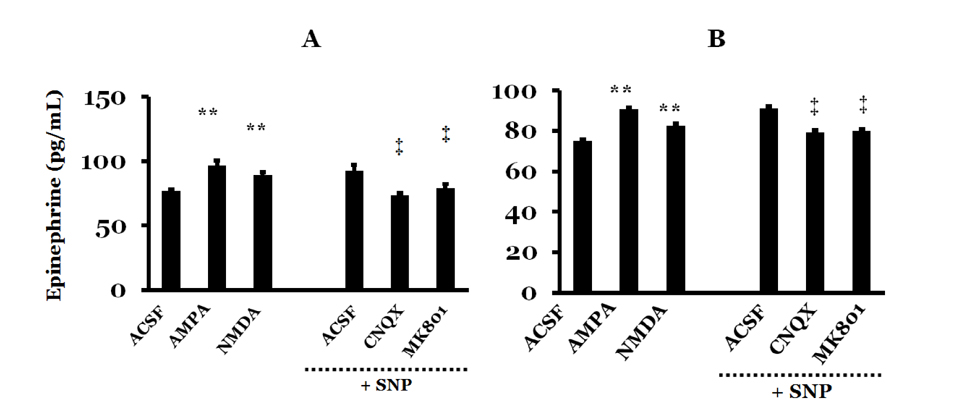
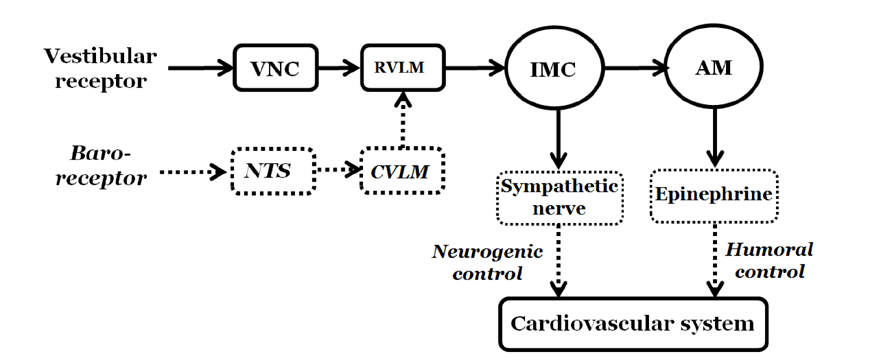
- Orthostatic hypotension (OH) is associated with symptoms including headache, dizziness, and syncope. The incidence of OH increases with age. Attenuation of the vestibulosympathetic reflex (VSR) is also associated with an increased incidence of OH. In order to understand the pathophysiology of OH, we investigated the physiological characteristics of the VSR in the disorder. We applied sodium nitroprusside (SNP) to conscious rats with sinoaortic denervation in order to induce hypotension. Expression of pERK in the intermediolateral cell column (IMC) of the T4~7 thoracic spinal regions, blood epinephrine levels, and blood pressure were evaluated following the administration of glutamate and/or SNP. SNP-induced hypotension led to increased pERK expression in the medial vestibular nucleus (MVN), rostral ventrolateral medullary nucleus (RVLM) and the IMC, as well as increased blood epinephrine levels. We co-administered either a glutamate receptor agonist or a glutamate receptor antagonist to the MVN or the RVLM. The administration of the glutamate receptor agonists, AMPA or NMDA, to the MVN or RVLM led to elevated blood pressure, increased pERK expression in the IMC, and increased blood epinephrine levels. Administration of the glutamate receptor antagonists, CNQX or MK801, to the MVN or RVLM attenuated the increased pERK expression and blood epinephrine levels caused by SNP-induced hypotension. These results suggest that two components of the pathway which maintains blood pressure are involved in the VSR induced by SNP. These are the neurogenic control of blood pressure via the RVLM and the humoral control of blood pressure via epinephrine release from the adrenal medulla.
Keyword
MeSH Terms
-
6-Cyano-7-nitroquinoxaline-2,3-dione
Adrenal Medulla
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Animals
Blood Pressure
Denervation
Dizocilpine Maleate
Dizziness
Epinephrine
Excitatory Amino Acid Antagonists
Glutamic Acid
Headache
Hypotension*
Hypotension, Orthostatic
Incidence
N-Methylaspartate
Nitroprusside
Rats*
Receptors, Glutamate
Reflex*
Spinal Cord Lateral Horn
Syncope
Vestibular Nuclei
6-Cyano-7-nitroquinoxaline-2,3-dione
Dizocilpine Maleate
Epinephrine
Excitatory Amino Acid Antagonists
Glutamic Acid
N-Methylaspartate
Nitroprusside
Receptors, Glutamate
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
Figure
Cited by 1 articles
-
Role of peripheral vestibular receptors in the control of blood pressure following hypotension
Guang-Shi Jin, Xiang-Lan Li, Yuan-Zhe Jin, Min Sun Kim, Byung Rim Park
Korean J Physiol Pharmacol. 2018;22(4):363-368. doi: 10.4196/kjpp.2018.22.4.363.
Reference
-
1. Schatz IJ, Bannister R, Freeman RL, Goetz CG. The definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. J Auton Nerv Syst. 1996; 58:123–124.2. Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002; 20:1675–1688.3. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006; 7:335–346.4. Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999; 519(Pt 1):1–10.5. Jiang X, Li LW, Lan Y, Yang YZ, Jin GS, Kim MS, Park BR, Jin YZ. Comparative analysis of vestibular receptor and baroreceptor inputs to the nucleus tractus solitarius following acute hypotension in conscious rats. Neurosci Lett. 2014; 563:70–74.6. Aoki M, Sakaida Y, Tanaka K, Mizuta K, Ito Y. Evidence for vestibular dysfunction in orthostatic hypotension. Exp Brain Res. 2012; 217:251–259.7. Wilson VJ, Jones GM. Mammalian vestibular physiology. New York: Plenum Press;1979.8. Park BR, Kim MS, Kim JH, Jin YZ. Effects of acute hypotension on neuronal activity in the medial vestibular nuclei of rats. Neuroreport. 2001; 12:3821–3824.9. Yates BJ. Vestibular influences on the sympathetic nervous system. Brain Res Brain Res Rev. 1992; 17:51–59.10. Kerman IA, Yates BJ, McAllen RM. Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R109–R117.11. Voustianiouk A, Kaufmann H, Diedrich A, Raphan T, Biaggioni I, Macdougall H, Ogorodnikov D, Cohen B. Electrical activation of the human vestibulo-sympathetic reflex. Exp Brain Res. 2006; 171:251–261.12. Yates BJ, Yamagata Y, Bolton PS. The ventrolateral medulla of the cat mediates vestibulosympathetic reflexes. Brain Res. 1991; 552:265–272.13. Yates BJ, Grélot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol. 1994; 267:R974–R983.14. Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res. 1994; 98:200–212.15. Lan Y, Yang YZ, Jiang X, Li LW, Jin GS, Kim MS, Park BR, Jin YZ. Additive role of the vestibular end organ and baroreceptors on the regulation of blood pressure in rats. Korean J Physiol Pharmacol. 2013; 17:367–373.16. Lan Y, Lu HJ, Jiang X, Li LW, Yang YZ, Jin GS, Park JY, Kim MS, Park BR, Jin YZ. Analysis of the baroreceptor and vestibular receptor inputs in the rostral ventrolateral medulla following hypotension in conscious rats. Korean J Physiol Pharmacol. 2015; 19:159–165.17. Lu HJ, Li MH, Li MZ, Park SE, Kim MS, Jin YZ, Park BR. Functional connections of the vestibulo-spino-adrenal axis in the control of blood pressure via the vestibulosympathetic reflex in conscious rats. Korean J Physiol Pharmacol. 2015; 19:427–434.18. Choi MA, Lee JH, Hwang JH, Choi SJ, Kim MS, Park BR. Signaling pathway of glutamate in the vestibular nuclei following acute hypotension in rats. Brain Res. 2008; 1229:111–117.19. Kim MS, Choi MA, Choi DO, Lee MY, Kim KY, Rhee JK, Jin YZ, Park BR. Asymmetric activation of extracellular signal-regulated kinase 1/2 in rat vestibular nuclei by unilateral labyrinthectomy. Brain Res. 2004; 1011:238–242.20. Morrison SF. Glutamate transmission in the rostral ventrolateral medullary sympathetic premotor pathway. Cell Mol Neurobiol. 2003; 23:761–772.21. Springell DA, Costin NS, Pilowsky PM, Goodchild AK. Hypotension and short-term anaesthesia induce ERK1/2 phosphorylation in autonomic nuclei of the brainstem. Eur J Neurosci. 2005; 22:2257–2270.22. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989; 29:261–265.23. Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol. 2003; 23:597–616.24. Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci Lett. 1994; 176:59–62.25. Wei S, Lei M, Tong M, Ding J, Han Q, Xiao M. Acute baroreceptor unloading evokes Fos expression in anesthetized rat brain. Brain Res Bull. 2008; 76:63–69.26. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY, USA: Academic Press;2007.27. Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res. 1997; 7:63–76.28. Holstein GR, Friedrich VL Jr, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience. 2011; 175:104–117.29. Szentagothai J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950; 13:395–407.30. Wilson VJ, Schor RH, Suzuki I, Park BR. Spatial organization of neck and vestibular reflexes acting on the forelimbs of the decerebrate cat. J Neurophysiol. 1986; 55:514–526.31. Normand H, Etard O, Denise P. Otolithic and tonic neck receptors control of limb blood flow in humans. J Appl Physiol (1985). 1997; 82:1734–1738.32. Biaggioni I, Costa F, Kaufmann H. Vestibular influences on autonomic cardiovascular control in humans. J Vestib Res. 1998; 8:35–41.33. Jiang X, Lan Y, Jin YZ, Park JY, Park BG, Ameer AN, Park BR. Effect of vestibulosympathetic reflex and baroreflex on expression of pERK in the nucleus tractus solitarius following acute hypotension in conscious rats. Korean J Physiol Pharmacol. 2014; 18:353–358.34. Doba N, Reis DJ. Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974; 34:9–18.35. Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002; 105:956–961.36. Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986; 78:1–5.37. Kim MS, Hyo Kim J, Kry D, Ae Choi M, Ok Choi D, Gon Cho B, Jin YZ, Ho Lee S, Park BR. Effects of acute hypotension on expression of cFos-like protein in the vestibular nuclei of rats. Brain Res. 2003; 962:111–121.38. Li XL, Nian B, Jin Y, Li LW, Jin GS, Kim MS, Park BR, Jin YZ. Mechanism of glutamate receptor for excitation of medial vestibular nucleus induced by acute hypotension. Brain Res. 2012; 1443:27–33.39. Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst. 1997; 66:154–162.40. Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull. 2000; 53:11–16.41. Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981; 112:195–203.42. Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994; 71:2087–2092.43. Holstein GR, Friedrich VL Jr, Martinelli GP. Projection neurons of the vestibulo-sympathetic reflex pathway. J Comp Neurol. 2014; 522:2053–2074.44. Ray CA, Hume KM. Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol (1985). 1998; 84:450–453.45. Sato T, Kawada T, Inagaki M, Shishido T, Sugimachi M, Sunagawa K. Dynamics of sympathetic baroreflex control of arterial pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R262–R270.46. Gotoh TM, Fujiki N, Matsuda T, Gao S, Morita H. Roles of baroreflex and vestibulosympathetic reflex in controlling arterial blood pressure during gravitational stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R25–R30.47. Bent LR, Bolton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2006; 174:701–711.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathways of Neurogenic and Humoral Control in the Vestibulosympathetic Reflex of Conscious Rats
- Role of peripheral vestibular receptors in the control of blood pressure following hypotension
- Role of Vestibulosympathetic Reflex on Orthostatic Hypotension in Rats
- Effect of Vestibulosympathetic Reflex and Baroreflex on Expression of pERK in the Nucleus Tractus Solitarius following Acute Hypotension in Conscious Rats
- Functional Connections of the Vestibulo-spino-adrenal Axis in the Control of Blood Pressure Via the Vestibulosympathetic Reflex in Conscious Rats