J Breast Cancer.
2011 Sep;14(3):223-228.
Comparison of CVF (Cyclophosphamide+Vinorelbine+5-Fluorouracil) and CMF (Cyclophosphamide+Methotrexate+5-Fluorouracil) Adjuvant Chemotherapy in Early Breast Cancer
- Affiliations
-
- 1Department of Surgery, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. breast@hanmail.net
- 2Department of Pathology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- 5Department of Surgery, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
Abstract
- PURPOSE
Our study aimed to evaluate the feasibility of adjuvant cyclophosphamide/vinorelbine/5-fluorourail (CVF) chemotherapy as an alternative to cyclophosphamide/methotrexate/5-fluorouracil (CMF) chemotherapy for treating early breast cancer.
METHODS
One hundred and forty-nine patients were randomly assigned to CMF or CVF adjuvant chemotherapy for treating their early stage breast cancer between September 2000 and December 2007. The disease-free survival (DFS), the overall survival (OS), and the toxicity profiles of both groups were compared.
RESULTS
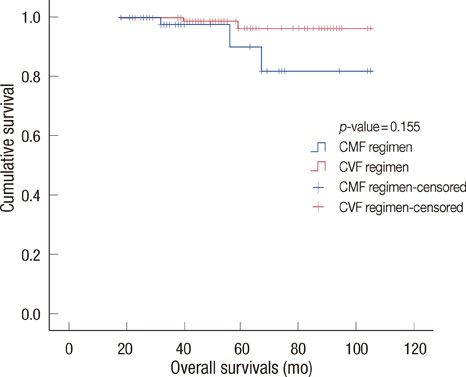
Sixty-seven patients underwent CMF chemotherapy whereas 82 patients underwent CVF chemotherapy. The DFS and OS were 88 months (95% confidence interval [CI], 76-101 months) and 94 months (95% CI, 83-104 months), respectively for the CMF group, and 97 months (95% CI, 93-101 months), and 101 months (95% CI, 98-104 months), respectively for the CVF group. However, those survival gains of the CVF group were not statistically significant (p-value=0.069 for the DFS and 0.99 for the OS). The CVF group showed a favorable toxicity profile in terms of the grade 3/4 hematologic toxicities as compared to that of the CMF group.
CONCLUSION
Clinical outcome of CVF chemotherapy was comparable to CMF with a favorable toxicity profiles. However, it is difficult to conclude the feasibility of CVF regimen because of small number of studied patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Shackney SE, McCormack GW, Cuchural GJ Jr. Growth rate patterns of solid tumors and their relation to responsiveness to therapy: an analytical review. Ann Intern Med. 1978. 89:107–121.
Article2. Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008. 8:790–797.
Article3. Heys SD, Sarkar T, Hutcheon AW. Primary docetaxel chemotherapy in patients with breast cancer: impact on response and survival. Breast Cancer Res Treat. 2005. 90:169–185.
Article4. Julka PK, Awasthy BS, Sharma DN, Gairola M, Rath GK. Paclitaxel-epirubicin in advanced breast cancer. J Assoc Physicians India. 1999. 47:499–502.5. Bonadonna G, Rossi A, Valagussa P, Veronesi U. Actual data on efficacy of surgical adjuvant chemotherapy with CMF in breast cancer. Arch Geschwulstforsch. 1978. 48:659.6. Tancini G, Bajetta E, Marchini S, Valagussa P, Bonadonna G, Veronesi U. Preliminary 3-year results of 12 versus 6 cycles of surgical adjuvant CMF in premenopausal breast cancer. Cancer Clin Trials. 1979. 2:285–292.7. Bonadonna G, Valagussa P, Rossi A, Tancini G, Brambilla C, Zambetti M, et al. Ten-year experience with CMF-based adjuvant chemotherapy in resectable breast cancer. Breast Cancer Res Treat. 1985. 5:95–115.
Article8. Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni L, et al. 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005. 330:217.
Article9. Krikorian A, Breillout F. Vinorelbine (Navelbine). A new semisynthetic vinca alkaloid. Onkologie. 1991. 14:7–12.
Article10. Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil Delgado MA, Kerbrat P, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993. 11:1245–1252.
Article11. Gasparini G, Caffo O, Barni S, Frontini L, Testolin A, Guglielmi RB, et al. Vinorelbine is an active antiproliferative agent in pretreated advanced breast cancer patients: a phase II study. J Clin Oncol. 1994. 12:2094–2101.
Article12. Ejlertsen B, Mouridsen HT, Langkjer ST, Andersen J, Sjöström J, Kjaer M, et al. Phase III study of intravenous vinorelbine in combination with epirubicin versus epirubicin alone in patients with advanced breast cancer: a Scandinavian Breast Group Trial (SBG9403). J Clin Oncol. 2004. 22:2313–2320.
Article13. Chua S, Smith IE, A'Hern RP, Coombes GA, Hickish TF, Robinson AC, et al. Neoadjuvant vinorelbine/epirubicin (VE) versus standard adriamycin/cyclophosphamide (AC) in operable breast cancer: analysis of response and tolerability in a randomised phase III trial (TOPIC 2). Ann Oncol. 2005. 16:1435–1441.
Article14. Weber BL, Vogel C, Jones S, Harvey H, Hutchins L, Bigley J, et al. Intravenous vinorelbine as first-line and second-line therapy in advanced breast cancer. J Clin Oncol. 1995. 13:2722–2730.
Article15. Elomaa I, Joensuu H, Blomqvist C. Vinorelbine, epirubicin and fluorouracil as first-line therapy in metastatic breast cancer--a phase II trial. Acta Oncol. 2003. 42:309–314.
Article16. Vici P, Di Lauro L, Sergi D, Foggi P, Viola G, Mottolese M, et al. A phase II trial of docetaxel and vinorelbine in patients with advanced breast cancer previously treated with anthracyclines. Oncology. 2008. 75:175–181.
Article17. Kerbrat P, Roché H, Bonneterre J, Veyret C, Lortholary A, Monnier A, et al. Epirubicin-vinorelbine vs FEC100 for node-positive, early breast cancer: French Adjuvant Study Group 09 trial. Br J Cancer. 2007. 96:1633–1638.
Article18. Cho H, Kwak K, Kim J, Sohn SC, Park K, Han S. Feasibility of concurrent adjuvant chemotherapy and radiotherapy after breast-conserving surgery in early breast cancer. J Korean Breast Cancer Soc. 2004. 7:289–293.
Article19. Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990. 8:1483–1496.
Article20. Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995. 13:2886–2894.
Article21. Slamon DJ, Press MF. Alterations in the TOP2A and HER2 genes: association with adjuvant anthracycline sensitivity in human breast cancers. J Natl Cancer Inst. 2009. 101:615–618.
Article22. Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N, et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005. 23:5166–5170.
Article23. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.24. Ardavanis A, Extra JM, Espié M, Cuvier C, Marty M. Phase II trial of a combination of vinorelbine, cyclophosphamide and 5-fluorouracil in the treatment of advanced breast cancer. In Vivo. 1998. 12:559–562.25. von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008. 100:542–551.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of adjuvant CMF(cyclophosphamide, methotrexate, 5-FU) chemotherapy of breast cancer
- Evaluation of the Survival Benefit of Different Chemotherapy Regimens in Patients with T1-2N0 Triple-Negative Breast Cancer
- The Incidence of Chemotherapy-induced Amenorrhea and Recovery in Young (<45-year-old) Breast Cancer Patients
- Cyclophosphamide, Methotrexate, and 5-Fluorouracil as Palliative Treatment for Heavily Pretreated Patients with Metastatic Breast Cancer: A Multicenter Retrospective Analysis
- Induction Chemotherapy for Breast Cancer: A case control study