J Breast Cancer.
2015 Sep;18(3):271-278. 10.4048/jbc.2015.18.3.271.
Evaluation of the Survival Benefit of Different Chemotherapy Regimens in Patients with T1-2N0 Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea. nohwoo@kcch.re.kr
- 2Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Cheil General Hospital and Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2407575
- DOI: http://doi.org/10.4048/jbc.2015.18.3.271
Abstract
- PURPOSE
This study aimed to evaluate the survival benefit of different adjuvant chemotherapy regimens in patients with T1-2N0 triple-negative breast cancer.
METHODS
Of 67,321 patients who were registered in the Korean Breast Cancer Society nationwide database between January 1999 and December 2008, 4,033 patients with T1-2N0 triple-negative breast cancer were included. The overall survival of patients who did not receive adjuvant chemotherapy was compared with those treated with adjuvant anthracycline and cyclophosphamide (AC), 5-fluorouracil, anthracycline, and cyclophosphamide (FAC), or cyclophosphamide, methotrexate, and 5-fluorouracil (CMF).
RESULTS
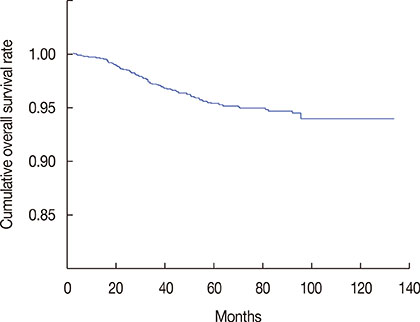
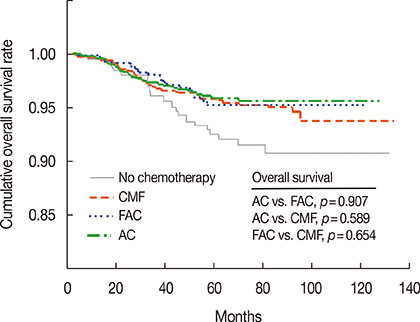
The median follow-up was 52.5 months. Chemotherapy was used in 87.4% of patients; it was used more commonly in patients with T2 tumors, those who were younger, had a higher histologic grade, and who showed lymphovascular invasion. The 5-year cumulative overall survival rate was 95.4%. Younger age, breast-conserving surgery, and adjuvant chemotherapy were significantly associated with improved overall survival. The 5-year cumulative overall survival rate of patients who did not receive adjuvant chemotherapy and those treated with AC, FAC, and CMF were 92.5%, 95.9%, 95.3%, and 95.9%, respectively. On multivariate analysis, the administration of any adjuvant chemotherapy regimen was significantly associated with improved overall survival (p=0.038). No significant difference in survival benefit was observed among the three different treatment groups.
CONCLUSION
A standard adjuvant chemotherapy regimen with the least drug-related toxicity might be a reasonable treatment for patients with T1-2N0 triple-negative breast cancer.
MeSH Terms
Figure
Reference
-
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717.2. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11:1135–1141.
Article3. Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011; 29:3366–3373.
Article4. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283.
Article5. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 Years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013; 382:1021–1028.
Article6. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010; 7:683–692.
Article7. Kim JE, Ahn HJ, Ahn JH, Yoon DH, Kim SB, Jung KH, et al. Impact of triple-negative breast cancer phenotype on prognosis in patients with stage I breast cancer. J Breast Cancer. 2012; 15:197–202.
Article8. Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012; 379:432–444.
Article9. Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013; 5:61–70.
Article10. Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol. 2012; 23:Suppl 6. vi40–vi45.
Article11. Hernandez-Aya LF, Gonzalez-Angulo AM. Adjuvant systemic therapies in breast cancer. Surg Clin North Am. 2013; 93:473–491.
Article12. Kim HA, Kim EK, Kim MS, Yu JH, Lee MR, Lee HK, et al. Association of human epidermal growth factor receptor 2 with radiotherapy resistance in patients with T1N0M0 breast cancer. J Breast Cancer. 2013; 16:266–273.
Article13. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368.
Article14. Colleoni M, Cole BF, Viale G, Regan MM, Price KN, Maiorano E, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010; 28:2966–2973.
Article15. Fisher B, Jeong JH, Anderson S, Wolmark N. Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst. 2004; 96:1823–1831.
Article16. Fisher B, Anderson S, Tan-Chiu E, Wolmark N, Wickerham DL, Fisher ER, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001; 19:931–942.
Article17. Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, Chia SK, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012; 18:2402–2412.18. Goldhirsch A, Coates AS, Colleoni M, Castiglione-Gertsch M, Gelber RD. International Breast Cancer Study Group. Adjuvant chemoendocrine therapy in postmenopausal breast cancer: cyclophosphamide, methotrexate, and fluorouracil dose and schedule may make a difference. J Clin Oncol. 1998; 16:1358–1362.19. Colleoni M, Price KN, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A. International Breast Cancer Study Group. Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil. Lancet. 1999; 354:130–131.
Article20. Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, et al. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003; 14:833–842.21. Hutchins LF, Green SJ, Ravdin PM, Lew D, Martino S, Abeloff M, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005; 23:8313–8321.22. Shulman LN, Cirrincione CT, Berry DA, Becker HP, Perez EA, O'Regan R, et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol. 2012; 30:4071–4076.
Article23. Martín M, Ruiz A, Ruiz Borrego M, Barnadas A, González S, Calvo L, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. J Clin Oncol. 2013; 31:2593–2599.24. Ozdemir N, Aksoy S, Zengin N, Altundag K. Taxanes in the adjuvant treatment of node-negative breast cancer patients. J BUON. 2012; 17:27–32.25. Lagha A, Chraiet N, Labidi S, Krimi S, Ayadi M, Gligorov J, et al. Impact of taxanes in the adjuvant setting of node-negative breast cancers. Bull Cancer. 2013; 100:465–471.26. Migdady Y, Sakr BJ, Sikov WM, Olszewski AJ. Adjuvant chemotherapy in T1a/bN0 HER2-positive or triple-negative breast cancers: application and outcomes. Breast. 2013; 22:793–798.
Article27. Olszewski AJ, Migdady Y, Boolbol SK, Klein P, Boachie-Adjei K, Sakr BJ, et al. Effects of adjuvant chemotherapy in HER2-positive or triple-negative pT1ab breast cancers: a multi-institutional retrospective study. Breast Cancer Res Treat. 2013; 138:215–223.
Article28. Ho AY, Gupta G, King TA, Perez CA, Patil SM, Rogers KH, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012; 118:4944–4952.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response to Paclitaxel in Node-positive Triple Negative Breast Cancer
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Which Patients Might Benefit from Postmastectomy Radiotherapy in Breast Cancer Patients with T1-2 Tumor and 1-3 Axillary Lymph Nodes Metastasis?
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors