J Pathol Transl Med.
2017 May;51(3):284-291. 10.4132/jptm.2017.02.16.
A Small Case Series of Intravascular Large B-Cell Lymphoma with Unexpected Findings: Subset of Cases with Concomitant Extravascular Central Nervous System (CNS) Involvement Mimicking Primary CNS Lymphoma
- Affiliations
-
- 1Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA. kate.poropatich@northwestern.edu
- KMID: 2392595
- DOI: http://doi.org/10.4132/jptm.2017.02.16
Abstract
- BACKGROUND
Intravascular large B-cell lymphoma (IVLBCL) is a rare type of extranodal lymphoma with growth mainly in the lumina of vessels. We studied a small series of IVLBCL and focused on its central nervous system (CNS) involvement.
METHODS
Searching the medical records of Northwestern Memorial Hospital, we identified five cases of IVLBCL from January 2007 to January 2015. Clinical information, hematoxylin and eosin stained histologic slides and immunohistochemistry studies were reviewed for all cases. Polymerase chain reaction (PCR) analysis for the immunoglobulin (Ig) heavy and light chain gene rearrangement was performed on all five cases.
RESULTS
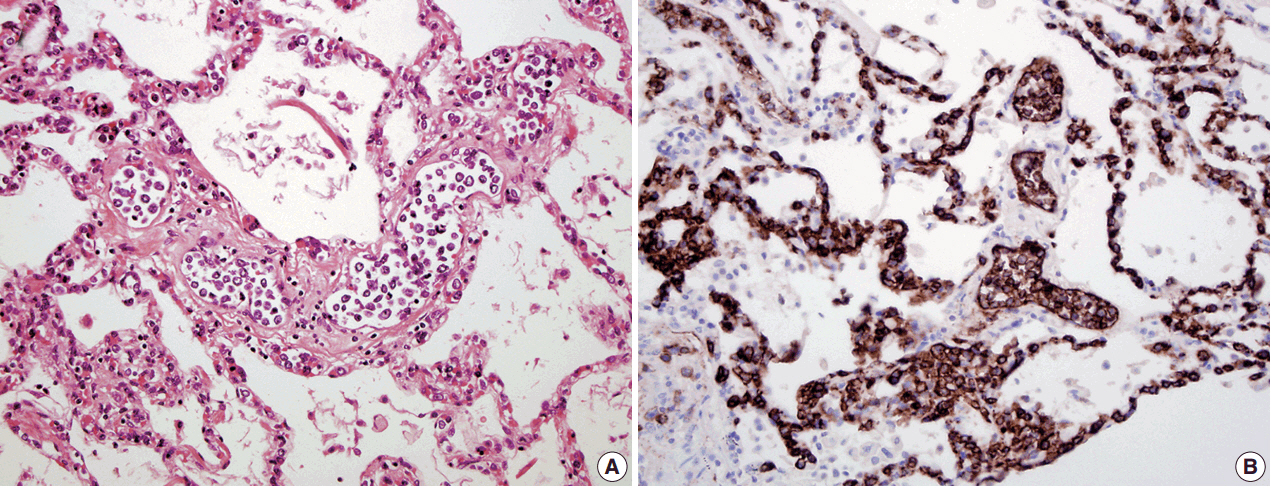
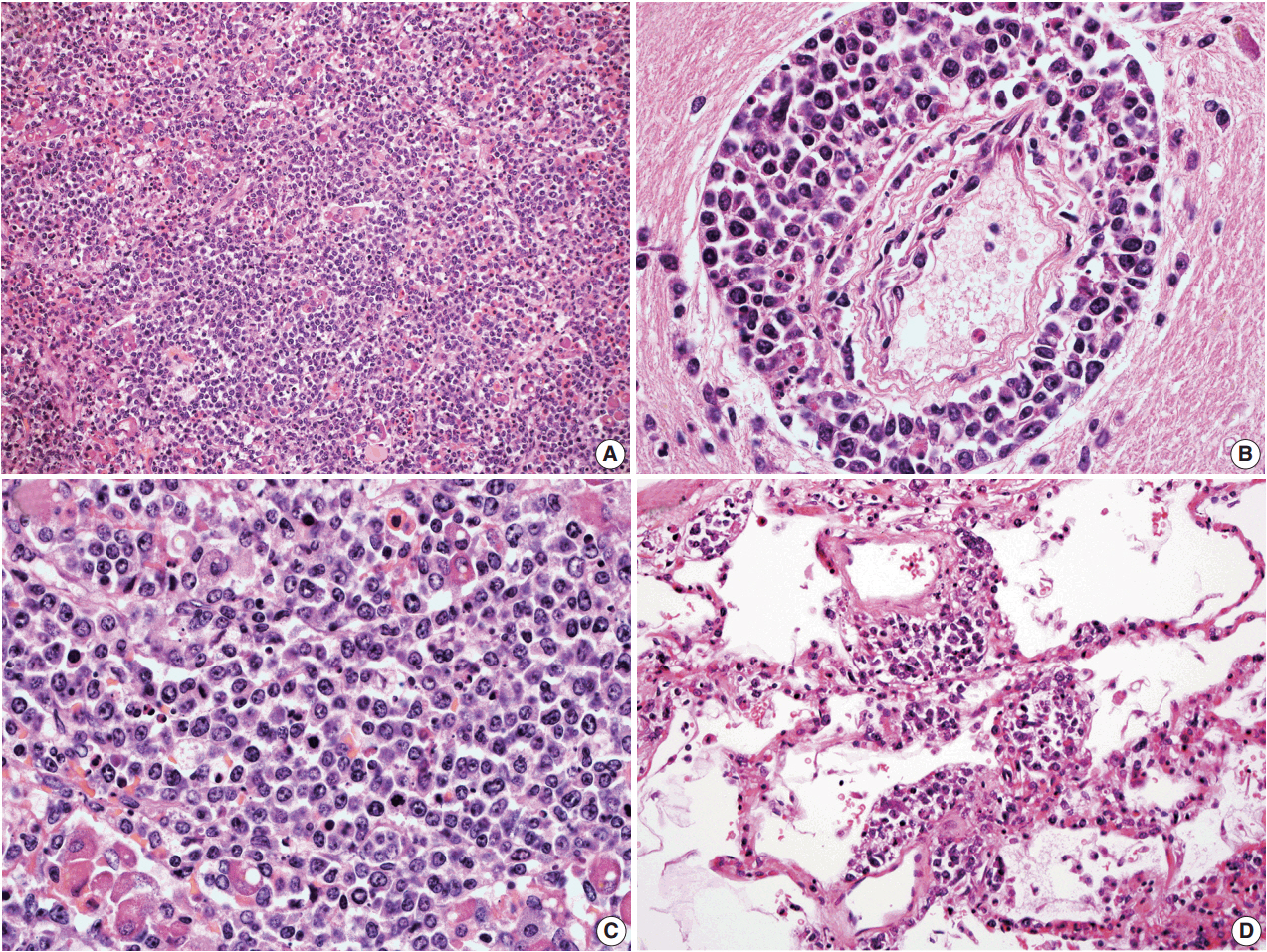
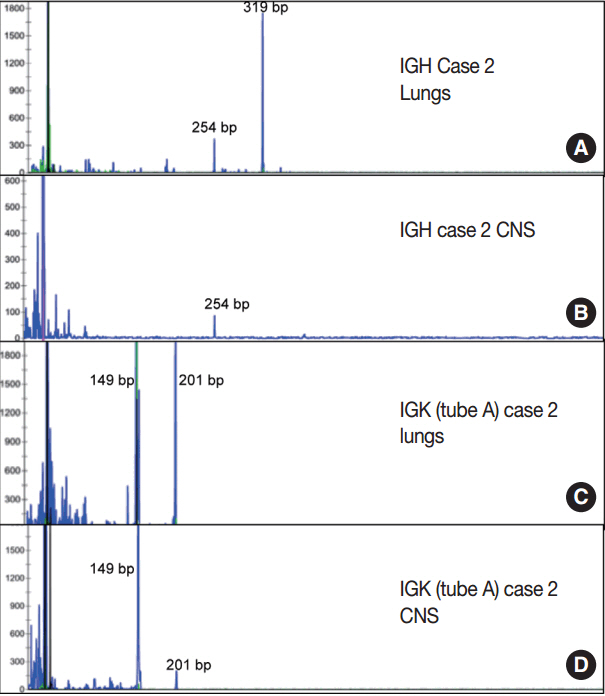
Three of the five cases of IVLBCL were autopsies. Patients' age ranged from 56 to 84. CNS involvement was present in two cases"”in both patients, the CNS involvement showed an extravascular pattern with confluent sheet-like formation. PCR analysis confirmed that in one case the systemic intravascular and CNS extravascular components were clonally identical.
CONCLUSIONS
In a small case series of IVLBCL, we observed that CNS involvement by IVLBCL often has an extravascular morphology, but is clonally identical to the intravascular counterpart by PCR analysis. As IVLBCL can have a rapidly progressing poor outcome, it should be kept in the differential diagnoses for patients presenting with lymphoma of the CNS. The presence of extravascular growth patterns in the CNS should not exclude IVLBCL as a diagnosis.
MeSH Terms
Figure
Reference
-
1. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–32.
Article2. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007; 109:478–85.
Article3. Fulling KH, Gersell DJ. Neoplastic angioendotheliomatosis: histologic, immunohistochemical, and ultrastructural findings in two cases. Cancer. 1983; 51:1107–18.
Article4. Ip M, Chan KW, Chan IK. Systemic inflammatory response syndrome in intravascular lymphomatosis. Intensive Care Med. 1997; 23:783–6.
Article5. Devlin T, Moll S, Hulette C, Morgenlander JC. Intravascular malignant lymphomatosis with neurologic presentation: factors facilitating antemortem diagnosis. South Med J. 1998; 91:672–6.6. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007; 25:3168–73.
Article7. Estalilla OC, Koo CH, Brynes RK, Medeiros LJ. Intravascular large B-cell lymphoma: a report of five cases initially diagnosed by bone marrow biopsy. Am J Clin Pathol. 1999; 112:248–55.
Article8. Fonkem E, Lok E, Robison D, Gautam S, Wong ET. The natural history of intravascular lymphomatosis. Cancer Med. 2014; 3:1010–24.
Article9. Glass J, Hochberg FH, Miller DC. Intravascular lymphomatosis: a systemic disease with neurologic manifestations. Cancer. 1993; 71:3156–64.
Article10. Fonkem E, Dayawansa S, Stroberg E, et al. Neurological presentations of intravascular lymphoma (IVL): meta-analysis of 654 patients. BMC Neurol. 2016; 16:9.
Article11. Wick MR, Mills SE, Scheithauer BW, Cooper PH, Davitz MA, Parkinson K. Reassessment of malignant “angioendotheliomatosis”: evidence in favor of its reclassification as “intravascular lymphomatosis”. Am J Surg Pathol. 1986; 10:112–23.12. Willemze R, Kerl H, Sterry W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997; 90:354–71.13. Yegappan S, Coupland R, Arber DA, et al. Angiotropic lymphoma: an immunophenotypically and clinically heterogeneous lymphoma. Mod Pathol. 2001; 14:1147–56.
Article14. Podduturi V, Guileyardo JM, Soto LR, Krause JR. A case series of clinically undiagnosed hematopoietic neoplasms discovered at autopsy. Am J Clin Pathol. 2015; 143:854–60.
Article15. Thomas CA, Guileyardo JM, Krause JR. An intravascular lymphoma with extravascular tendencies. Proc (Bayl Univ Med Cent). 2014; 27:341–3.
Article16. Demirer T, Dail DH, Aboulafia DM. Four varied cases of intravascular lymphomatosis and a literature review. Cancer. 1994; 73:1738–45.
Article17. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 39-1986. A 66-year-old woman with fever, fluctuating neurologic signs, and negative blood cultures. N Engl J Med. 1986; 315:874–85.18. Ferry JA, Harris NL, Picker LJ, et al. Intravascular lymphomatosis (malignant angioendotheliomatosis): a B-cell neoplasm expressing surface homing receptors. Mod Pathol. 1988; 1:444–52.19. Imai H, Kajimoto K, Taniwaki M, et al. Intravascular large B-cell lymphoma presenting with mass lesions in the central nervous system: a report of five cases. Pathol Int. 2004; 54:231–6.
Article20. Takeuchi Y, Hashizume Y, Hoshiyama M, Hirose Y, Takahashi A. An autopsy case of intravascular malignant lymphomatosis with intracranial lymphomatous mass lesions. Rinsho Shinkeigaku. 1995; 35:158–63.21. Beal MF, Fisher CM. Neoplastic angioendotheliosis. J Neurol Sci. 1982; 53:359–75.
Article22. Fonseca VR, Espada E, Geraldes R, et al. Synchronous brain and intravascular B-cell lymphoma after remission of an adult hemophagocytic syndrome. Clin Case Rep. 2016; 4:327–30.
Article23. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.
Article24. Murase T, Nakamura S, Kawauchi K, et al. An Asian variant of intravascular large B-cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B-cell lymphoma associated with haemophagocytic syndrome. Br J Haematol. 2000; 111:826–34.
Article25. Baehring JM, Longtine J, Hochberg FH. A new approach to the diagnosis and treatment of intravascular lymphoma. J Neurooncol. 2003; 61:237–48.26. Sugita Y, Muta H, Ohshima K, et al. Primary central nervous system lymphomas and related diseases: Pathological characteristics and discussion of the differential diagnosis. Neuropathology. 2016; 36:313–24.
Article27. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004; 127:173–83.28. Kinoshita M, Izumoto S, Hashimoto N, et al. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol. 2008; 25:73–8.
Article29. Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009; 1788:842–57.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Salvage Treatment for Isolated Brain Parenchymal Relapse due to Diffuse Large B Cell Lymphoma
- Asian variant of intravascular large B-cell lymphoma: a comparison of clinical features based on involvement of the central nervous system
- A Case of Panhypopituitarism and Central Diabetes Insipidus Caused by Primary Central Nervous System Lymphoma
- A case of peripheral T-cell lymphoma with central nervous system and bilateral pulmonary involvement
- Primary Central Nervous System Lymphoma Associated with Systemic Lymphoma: Case Report