Ann Surg Treat Res.
2017 Oct;93(4):209-216. 10.4174/astr.2017.93.4.209.
The optimal duration of ischemic preconditioning for renal ischemia-reperfusion injury in mice
- Affiliations
-
- 1Department of Clinical Research, Daejeon St. Mary's Hospital, Daejeon, Korea.
- 2Department of Surgery, Daejeon St. Mary's Hospital, Daejeon, Korea. jjungyong@catholic.ac.kr
- 3Department of Internal Medicine, Daejeon St. Mary's Hospital, Daejeon, Korea.
- 4Department of Surgery, Uijeongbu St. Mary's Hospital, Uijeongbu, Korea.
- 5Department of Surgery, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2392371
- DOI: http://doi.org/10.4174/astr.2017.93.4.209
Abstract
- PURPOSE
The aim of the present study was to investigate the protective effects of ischemic preconditioning for different periods of time and to elucidate the optimal safe ischemic preconditioning time for renal ischemia-reperfusion (I/R) injury in mice.
METHODS
A total of 25 male C57BL/6 mice were randomly divided into 5 groups (sham, I/R, ischemic preconditioning [IP]-3, IP-5, and IP-7 groups), in which the kidney was preconditioned with IP of various durations and then subjected to I/R injury (the last 3 groups). To induce renal ischemia, the left renal pedicle was occluded with a nontraumatic microaneurysm clamp for 30 minutes followed by reperfusion for 24 hours. The effects of IP on renal I/R injury were evaluated in terms of renal function, tubular necrosis, apoptotic cell death and inflammatory cytokines.
RESULTS
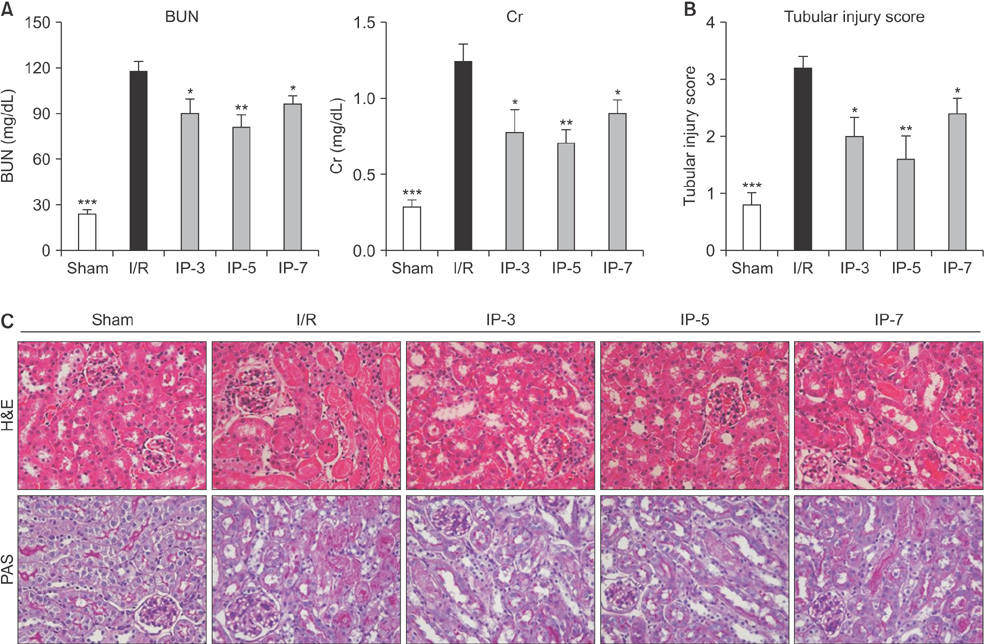
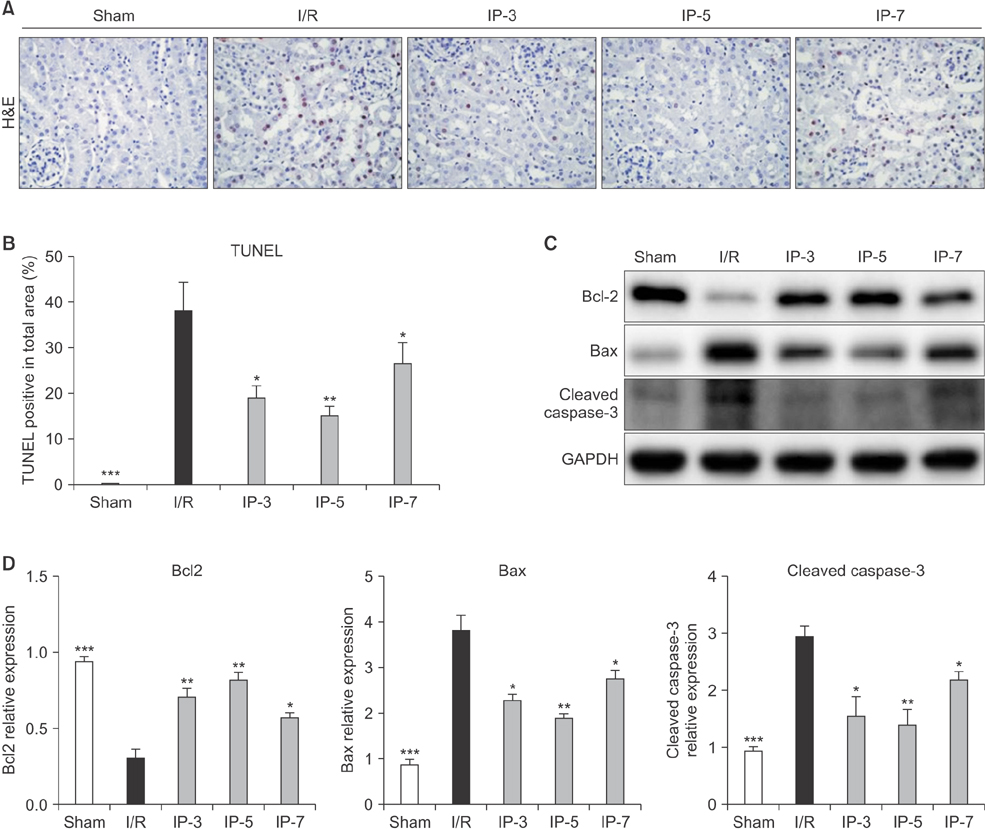
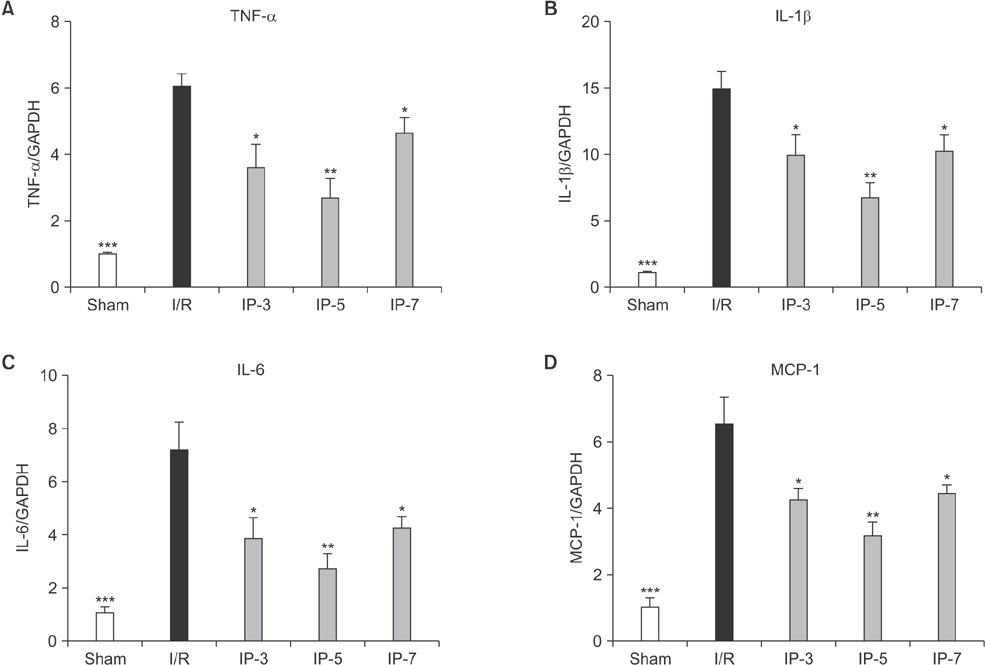
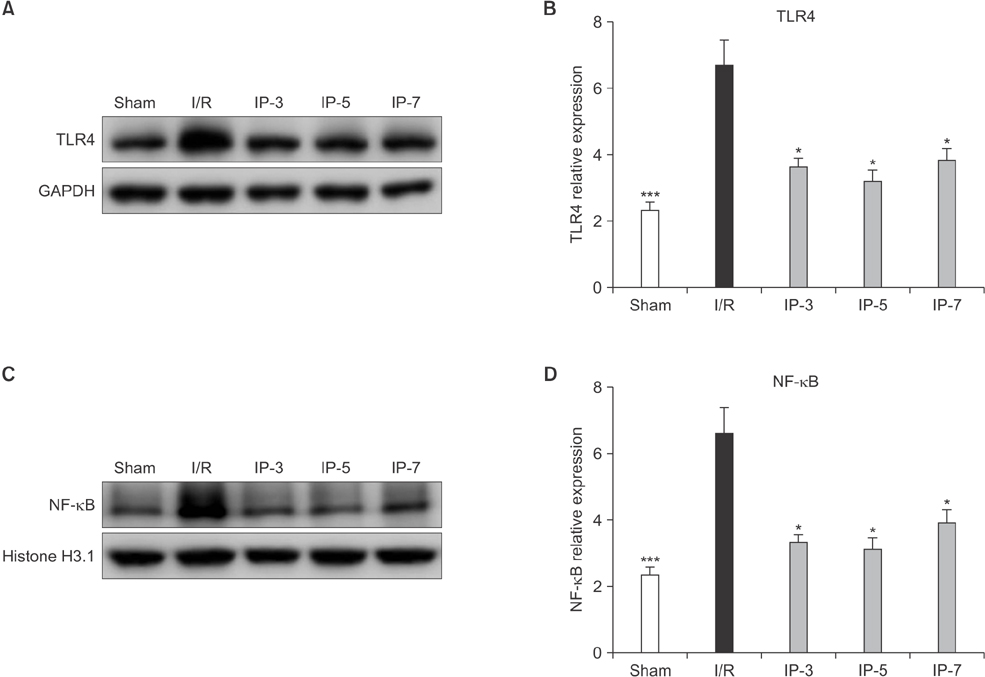
Results indicated that BUN and creatinine (Cr) levels increased significantly in the I/R group, but the elevations were significantly lower in IP groups, especially in the IP-5 group. Histological analysis revealed that kidney injury was markedly decreased in the IP-5 group compared with the I/R group, as evidenced by reduced renal necrosis/apoptosis. In addition, IP significantly inhibited gene expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (monocyte chemoattractant protein-1). Western blot analysis indicated that the expression levels of Toll-like receptor 4 (TLR4) and nuclear factor-kappa B (NF-κB) were upregulated in the I/R group, while expression was inhibited in the IP groups.
CONCLUSION
Five-minute IP had the greatest protective effect against I/R injury.
MeSH Terms
Figure
Reference
-
1. Yoshida T, Kumagai H, Kohsaka T, Ikegaya N. Relaxin protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013; 305:F1169–F1176.2. Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001; 281:F948–F957.3. Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009; 296:H1–H12.4. Valeur HS, Valen G. Innate immunity and myocardial adaptation to ischemia. Basic Res Cardiol. 2009; 104:22–32.5. Zhao H, Perez JS, Lu K, George AJ, Ma D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014; 306:F801–F811.6. Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010; 6:224–235.7. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007; 117:2847–2859.8. Jiang SH, Liu CF, Zhang XL, Xu XH, Zou JZ, Fang Y, et al. Renal protection by delayed ischaemic preconditioning is associated with inhibition of the inflammatory response and NF-kappaB activation. Cell Biochem Funct. 2007; 25:335–343.9. Jia RP, Xie JJ, Luo FY, Zhu JG. Ischemic preconditioning improves rat kidney allograft function after ischemia/reperfusion injury: the role of tumor necrosis factor-alpha. Transplant Proc. 2008; 40:3316–3320.10. Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006; 17:3115–3123.11. Chen X, Liu X, Wan X, Wu Y, Chen Y, Cao C. Ischemic preconditioning attenuates renal ischemia-reperfusion injury by inhibiting activation of IKKbeta and inflammatory response. Am J Nephrol. 2009; 30:287–294.12. Wever KE, Menting TP, Rovers M, van der, Rongen GA, Masereeuw R, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012; 7:e32296.13. Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012; 303:F1487–F1494.14. Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995; 76:73–81.15. Peralta C, Closa D, Xaus C, Gelpi E, Rosello-Catafau J, Hotter G. Hepatic preconditioning in rats is defined by a balance of adenosine and xanthine. Hepatology. 1998; 28:768–773.16. Peralta C, Hotter G, Closa D, Gelpi E, Bulbena O, Rosello-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997; 25:934–937.17. Hotter G, Closa D, Prados M, Fernandez-Cruz L, Prats N, Gelpi E, et al. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996; 222:27–32.18. Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci U S A. 1995; 92:4666–4670.19. Torras J, Herrero-Fresneda I, Lloberas N, Riera M, Ma Cruzado J, Ma Grinyó J. Promising effects of ischemic preconditioning in renal transplantation. Kidney Int. 2002; 61:2218–2227.20. Ferencz A, Racz B, Gasz B, Benko L, Jancso G, Kurthy M, et al. Intestinal ischemic preconditioning in rats and NF-kappaB activation. Microsurgery. 2006; 26:54–57.21. Liu M, Gu M, Xu D, Lv Q, Zhang W, Wu Y. Protective effects of Toll-like receptor 4 inhibitor eritoran on renal ischemia-reperfusion injury. Transplant Proc. 2010; 42:1539–1544.22. Bergler T, Hoffmann U, Bergler E, Jung B, Banas MC, Reinhold SW, et al. Toll-like receptor 4 in experimental kidney transplantation: early mediator of endogenous danger signals. Nephron Exp Nephrol. 2012; 121:e59–e70.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hypoxic-Preconditioning on the Reperfusion-Induced Arrhythmias in the Cat Hearts
- Renal Protective Effects of Opposite Renal Ischemic Preconditioning against Renal Ischemic Reperfusion Injury in Mice
- Effect of Ischemic Preconditioning on Catecholamine Release from the Isolated, Ischemic Reperfused Hearts of Rats
- Alteration of NF -kappa kappaB in Ischemic -reperfused Anterior Tibialis and Soleus Muscles of Rats
- The Effect of Ischemic Preconditioning on the Expression of Serum Cytokines after 2 Hours Ischemia and Timely Reperfusion in the Hindlimb of Mice