Allergy Asthma Respir Dis.
2016 Sep;4(5):354-359. 10.4168/aard.2016.4.5.354.
Pediatric adverse drug reactions collected by an electronic reporting system in a single tertiary university hospital
- Affiliations
-
- 1Department of Pharmacy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 3Environmental Health Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Allergy and Clinical Immunology, Asan Regional Pharmacovigilance Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2391925
- DOI: http://doi.org/10.4168/aard.2016.4.5.354
Abstract
- PURPOSE
The incidence of adverse drug reactions (ADRs) is increasing. However, studies on the prevalence of ADRs in children are rare. The aim of this study was to investigate the causative drugs and clinical features of ADRs for children in a tertiary university hospital of Korea.
METHODS
We retrospectively collected ADRs by a computerized self-reporting system in Asan Medical Center. ADRs of children under the age 18 were collected from January 2005 to August 2015, and we analyzed only ADRs containing current symptoms among total ADR data.
RESULTS
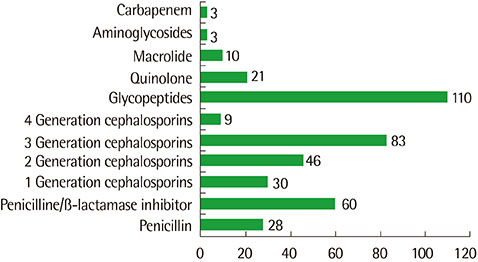
A total of 1,408 ADR cases were reported, There were 764 male (54.3%) and 644 female patients (45.7%), and the mean age was 11.5±5.8 years (range, 0-18 years). Antibiotics (n=479, 34.0%) were the most common causative drugs, followed by tramadol (n=173, 12.3%), nonsteroidal anti-inflammatory agents (NSAIDs) and acetylsalicylic acid (n=103, 7.3%), narcotics (n=91, 6.5%), antineoplastics (n=87, 6.2%), and sedatives (n=82, 5.8%). The most common clinical features were skin manifestations (n=500, 34.4%). Gastrointestinal symptoms (n=435, 29.9%) were the second most common clinical features, followed by neuropsychiatric symptoms (n=155, 10.7%) and respiratory symptoms (n=123, 8.5%). Among antibiotics, glycopeptides (n=110, 23.0%), third-generation cephalosporins (n=83, 17.3%), and penicillin/β-lactamase inhibitors (n=60, 12.7%) were the most frequently reported causative drugs.
CONCLUSION
Antibiotics were the most reported common causative drugs of ADRs in children, followed by tramadol, NSAID, and narcortics. Compared with adults, the prevalence of contrast medium-induced ADR was lower in children with a higher prevalence of sedative-associated ADR. Greater attention to possible ADRs in children is needed among medical personnel.
Keyword
MeSH Terms
-
Adult
Anti-Bacterial Agents
Anti-Inflammatory Agents, Non-Steroidal
Antineoplastic Agents
Aspirin
Cephalosporins
Child
Chungcheongnam-do
Drug-Related Side Effects and Adverse Reactions*
Female
Glycopeptides
Humans
Hypnotics and Sedatives
Incidence
Korea
Male
Narcotics
Prevalence
Retrospective Studies
Skin Manifestations
Tramadol
Anti-Bacterial Agents
Anti-Inflammatory Agents, Non-Steroidal
Antineoplastic Agents
Aspirin
Cephalosporins
Glycopeptides
Hypnotics and Sedatives
Narcotics
Tramadol
Figure
Cited by 2 articles
-
Clinical implication of adverse drug reaction surveillance in children
Eun Hee Chung
Allergy Asthma Respir Dis. 2016;4(5):309-310. doi: 10.4168/aard.2016.4.5.309.Analysis of pediatric adverse drug reactions reported to regional pharmacovigilance center of a single university hospital
Do-Woo Kim, Yun-Chang Choi, Young-Seok Lee, Young-Hee Nam, Jin-A Jung
Allergy Asthma Respir Dis. 2018;6(5):263-269. doi: 10.4168/aard.2018.6.5.263.
Reference
-
1. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972; 498:1–25.2. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998; 279:1200–1205.
Article3. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004; 329:15–19.
Article4. Bowman L, Carlstedt BC, Black CD. Incidence of adverse drug reactions in adult medical inpatients. Can J Hosp Pharm. 1994; 47:209–216.5. Lundkvist J, Jönsson B. Pharmacoeconomics of adverse drug reactions. Fundam Clin Pharmacol. 2004; 18:275–280.
Article6. Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998-2005. Arch Intern Med. 2007; 167:1752–1759.
Article7. Demoly P, Bousquet J. Epidemiology of drug allergy. Curr Opin Allergy Clin Immunol. 2001; 1:305–310.
Article8. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ. 1998; 316:1295–1298.9. Kim MH, Jung HY, Sohn MK, Kim SE, Lee YW, Park JW, et al. Clinical features of adverse drug reactions in a tertiary care hospital in Korea. Korean J Asthma Allergy Clin Immunol. 2008; 28:35–39.10. Kim MG, Kang HR, Kim JH, Ju YS, Park SH, Hwang YIl, et al. Analysis of adverse drug reactions collected by an electronic reporting system in a single hospital. Korean J Med. 2009; 77:601–609.11. Kim JE, Kyun JO, Jin SM, Lee YH, Kim JH, Lee HY, et al. Adverse drug reactions in elderly inpatients: comparison with younger patients. Korean J Asthma Allergy Clin Immunol. 2010; 30:216–221.12. Choi JH, Shin YS, Suh CH, Nahm DH, Park HS. The frequency of adverse drug reactions in a tertiary care hostpital in Korea. Korean J Med. 2004; 67:290–296.13. Rew SY, Koh YI, Shin HY, Park SH, Ryu SH, Kim HN, et al. Reporting and clinical features of adverse drug reactions from a single university hospital. Korean J Asthma Allergy Clin Immunol. 2011; 31:184–191.14. Kos M, Wertheimer AI, Mrhar A. Satisfaction with pharmacotherapy for approved and off-label indications: a Delphi study. Ann Pharmacother. 2005; 39:649–654.
Article15. Geum MJ, Park EH, Ko JH, Kim SH, Kim SE, Seok HJ. Case analysis of adverse drug reaction for children in Yonsei University Healthcare System. J Korean Soc Health Syst Pharm. 2011; 28:253–261.
Article16. Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000; 106:633–644.17. Malviya S, Voepel-Lewis T, Prochaska G, Tait AR. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics. 2000; 105:E42.
Article18. Kim HM, Seong JM, Yang BR, Jin XM, Choi NK, Lee J, et al. Analysis of adverse events reporting patterns and signal detection for pediatric patients in the Korean spontaneous reporting data. Korean Soc Pharmacoepidemiol Risk Manage. 2012; 5:40–45.19. Ferrajolo C, Capuano A, Trifirò G, Moretti U, Rossi F, Santuccio C. Pediatric drug safety surveillance in Italian pharmacovigilance network: an overview of adverse drug reactions in the years 2001 - 2012. Expert Opin Drug Saf. 2014; 13:Suppl 1. S9–S20.
Article20. Park GM, Seo JH, Kim HY, Yu J, Hong SJ. Four cases of drug allergy caused by non-steroidal anti-inflammatory drugs in children. Pediatr Allergy Respir Dis. 2011; 21:344–349.
Article21. Mirakian R, Ewan PW, Durham SR, Youlten LJ, Dugué P, Friedmann PS, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009; 39:43–61.
Article22. Jung JH, Kim JT, Yoo KY. Assessment of pediatric adverse drug reaction reports. J Korean Soc Health Syst Pharm. 2013; 30:108–118.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Change of the Etiology of Drug Induced Anaphylaxis in a Korean Tertiary Care Hospital
- Frequency and Pattern of Noninfectious Adverse Transfusion Reactions at a Tertiary Care Hospital in Korea
- Adverse Drug Reactions in Adult Patients Visiting an Emergency Department: Based on Spontaneous Reporting System
- Spontaneous Reporting of Adverse Drug Reactions through Electronic Submission from Regional Society Healthcare Professionals in Korea
- Active Participation in Reporting Adverse Drug Reactions