Ann Lab Med.
2016 Jan;36(1):36-41. 10.3343/alm.2016.36.1.36.
Frequency and Pattern of Noninfectious Adverse Transfusion Reactions at a Tertiary Care Hospital in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. hyunok1019@yuhs.ac
- 2Department of Laboratory Medicine, The Armed Forces Capital Hospital, Seongnam, Korea.
- KMID: 2373495
- DOI: http://doi.org/10.3343/alm.2016.36.1.36
Abstract
- BACKGROUND
Although transfusion is a paramount life-saving therapy, there are multiple potential significant risks. Therefore, all adverse transfusion reaction (ATR) episodes require close monitoring. Using the computerized reporting system, we assessed the frequency and pattern of non-infectious ATRs.
METHODS
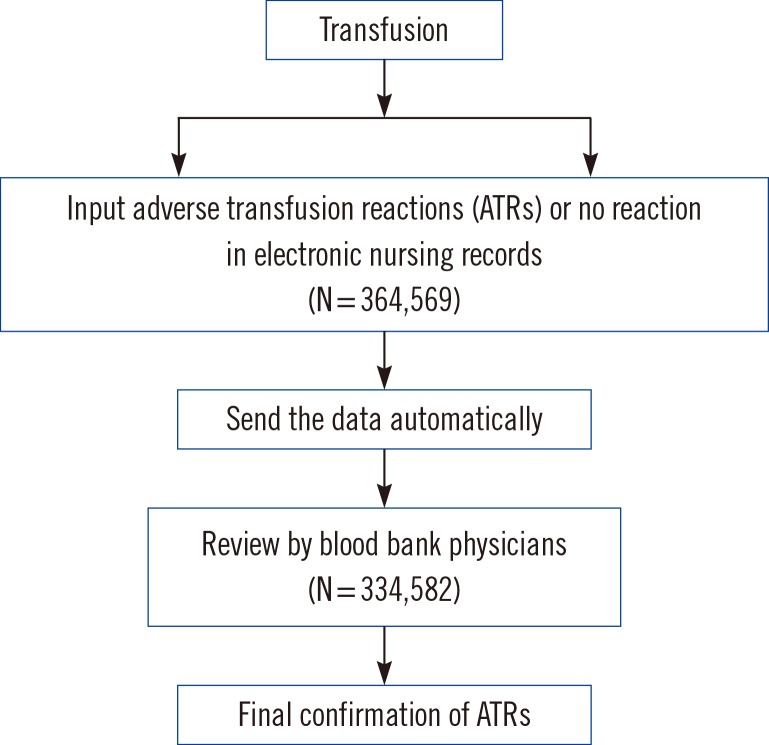
We analyzed two-year transfusion data from electronic medical records retrospectively. From March 2013 to February 2015, 364,569 units of blood were transfused. Of them, 334,582 (91.8%) records were identified from electronic nursing records. For the confirmation of ATRs by blood bank physicians, patients' electronic medical records were further evaluated.
RESULTS
According to the nursing records, the frequency of all possible transfusion-related events was 3.1%. After the blood bank physicians' review, the frequency was found to be 1.2%. The overall frequency of febrile non-hemolytic transfusion reactions (FNHTRs) to red blood cells (RBCs), platelet (PLT) components, and fresh frozen plasmas (FFPs) were 0.9%, 0.3%, and 0.2%, respectively, and allergic reactions represented 0.3% (RBCs), 0.9% (PLTs), and 0.9% (FFPs), respectively. The pre-storage leukocyte reduction significantly decreased the frequency of FNHTRs during the transfusion of RBCs (P<0.01) or PLTs (Pfalling dots0.01).
CONCLUSIONS
The frequency of FNHTRs, allergic reactions, and "no reactions" were 22.0%, 17.0%, and 60.7%, respectively. Leukocyte-reduction was associated with a lower rate of FNHTRs, but not with that of allergic reactions. The development of an effective electronic reporting system of ATRs is important in quantifying transfusion-related adverse events. This type of reporting system can also accurately identify the underlying problems and risk factors to further the quality of transfusion care for patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Case Report of Transfusion-associated Circulatory Overload
Daewon Kim, Hyunjin Nah, Sinyoung Kim, Hyun Ok Kim
Lab Med Online. 2019;9(1):30-34. doi: 10.3343/lmo.2019.9.1.30.
Reference
-
1. Sahu S, Hemlata , Verma A. Adverse events related to blood transfusion. Indian J Anaesth. 2014; 58:543–551. PMID: 25535415.
Article2. Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009; 108:759–769. PMID: 19224780.
Article3. Kato H, Nakayama T, Uruma M, Okuyama Y, Handa M, Tomiyama Y, et al. A retrospective observational study to assess adverse transfusion reactions of patients with and without prior transfusion history. Vox Sang. 2015; 108:243–250. PMID: 25536173.
Article4. Tinegate H, Birchall J, Gray A, Haggas R, Massey E, Norfolk D, et al. Guideline on the investigation and management of acute transfusion reactions. Prepared by the BCSH Blood Transfusion Task Force. Br J Haematol. 2012; 159:143–153. PMID: 22928769.
Article5. Fry JL, Arnold DM, Clase CM, Crowther MA, Holbrook AM, Traore AN, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010; 50:1722–1730. PMID: 20345566.
Article6. Mazzei CA, Popovsky MA, Kopko PM. Noninfectious complications of blood transfusion. In : Mark K, Brenda J, Christophjer D, Connie M, editors. Technical manual. 18th ed. Bethesda, MD: American Association of Blood Banks;2014. p. 665–696.7. Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention Atlanta GA, USA. National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol c.2.1.3. Updated on August 2014. http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf.8. Yeh SP, Chang CW, Chen JC, Yeh WC, Chen PC, Chuang SJ, et al. A well-designed online transfusion reaction reporting system improves the estimation of transfusion reaction incidence and quality of care in transfusion practice. Am J Clin Pathol. 2011; 136:842–847. PMID: 22095368.
Article9. de Vries RR, Faber JC, Strengers PF. Board of the International Haemovigilance Network. Haemovigilance: an effective tool for improving transfusion practice. Vox Sang. 2011; 100:60–67. PMID: 21175656.
Article10. Uhlmann EJ, Isgriggs E, Wallhermfechtel M, Goodnough LT. Prestorage universal WBC reduction of RBC units does not affect the incidence of transfusion reactions. Transfusion. 2001; 41:997–1000. PMID: 11493730.
Article11. Yazer MH, Podlosky L, Clarke G, Nahirniak SM. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004; 44:10–15. PMID: 14692961.
Article12. Bilgin YM, van de Watering LM, Brand A. Clinical effects of leucoreduction of blood transfusions. Neth J Med. 2011; 69:441–450. PMID: 22058263.13. Harvey AR, Basavaraju SV, Chung KW, Kuehnert MJ. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015; 55:709–718.
Article14. Wang RR, Triulzi DJ, Qu L. Effects of prestorage vs poststorage leukoreduction on the rate of febrile nonhemolytic transfusion reactions to platelets. Am J Clin Pathol. 2012; 138:255–259. PMID: 22904138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of Adverse Reaction to Transfusion in Pediatric Patients
- Incidence of Adverse Transfusion Reactions from an Institutional Hemovigilance System: A Single Center Study
- The frequency of adverse drug reactions in a tertiary care hostpital in Korea
- Incidence and Analysis of Adverse Transfusion Reactions at a Single Children’s Hospital
- Reporting System of Transfusion Adverse Reaction Using Electronic Medical Records Data