J Korean Neurosurg Soc.
2017 Jul;60(4):404-416. 10.3340/jkns.2016.1010.008.
Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury?
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. srjeon@amc.seoul.kr
- 2Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2387878
- DOI: http://doi.org/10.3340/jkns.2016.1010.008
Abstract
OBJECTIVE
Functional and neural tissue recovery has been reported in many animal studies conducted with stem cells. However, the combined effect of cytokines and stem cells has not yet been adequately researched. Here, we analyzed the additive effects of granulocyte colony-stimulating factor (GCSF) on adipose-derived stem cells (ADSCs) infusion in the treatment of acute spinal cord injury (SCI) in rats.
METHODS
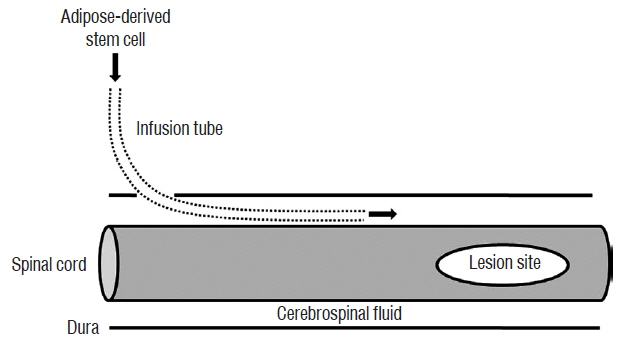
Four days after intrathecal infusion tubes implantation in Sprague-Dawley rats, SCI was induced with an infinite horizon impactor. In the Sham group (n=5), phosphate-buffered saline was injected 3, 7, and 14 days after SCI. GCSF, ADSCs, and ADSCs with GCSF were injected at the same time in the GCSF (n=8), ADSC (n=8), and ADSC+GCSF groups (n=7), respectively.
RESULTS
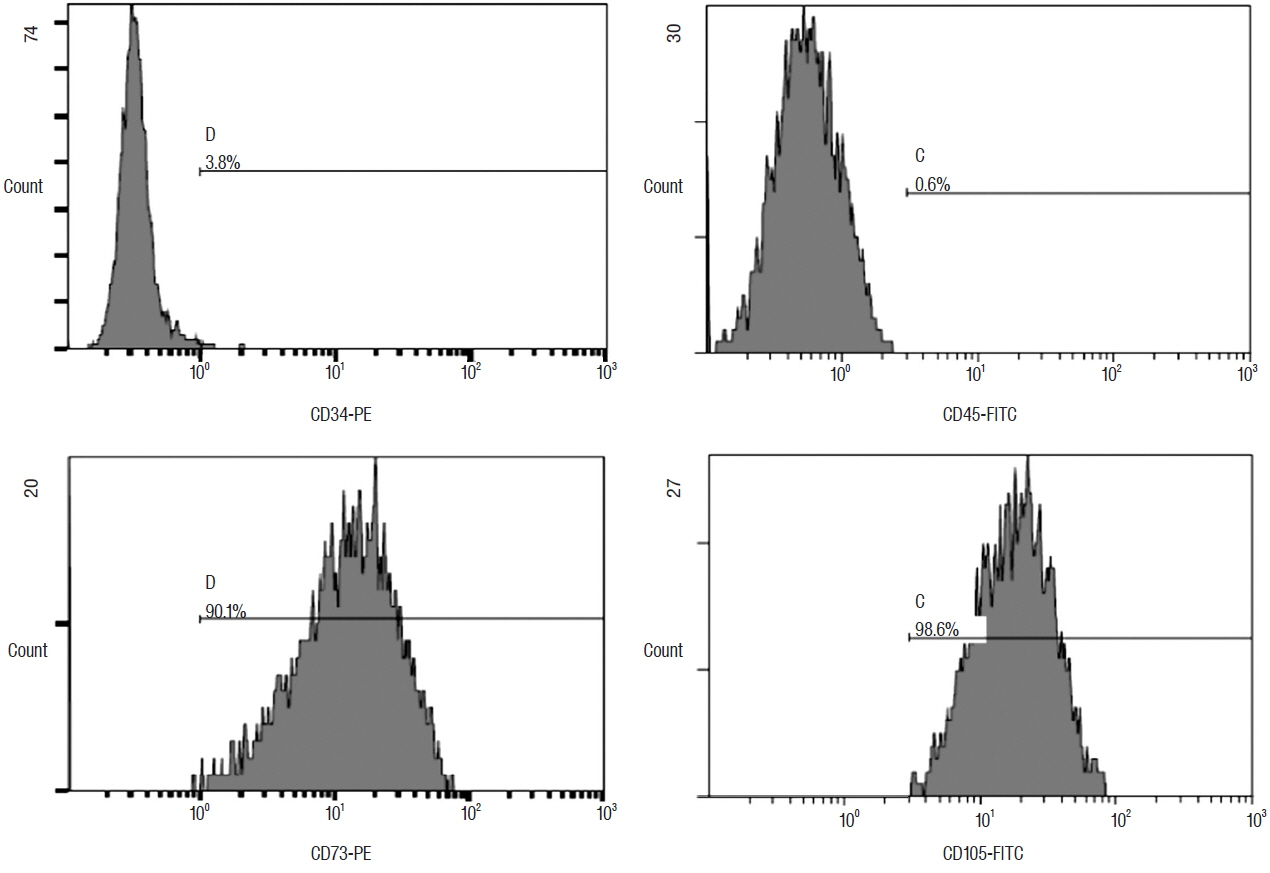
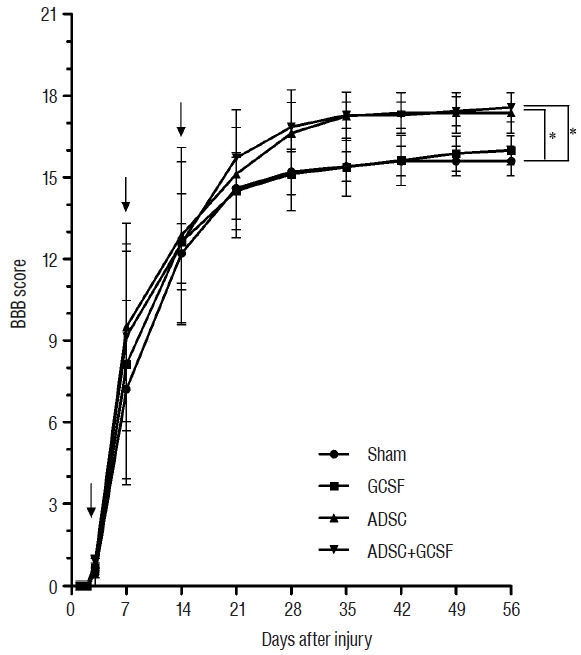
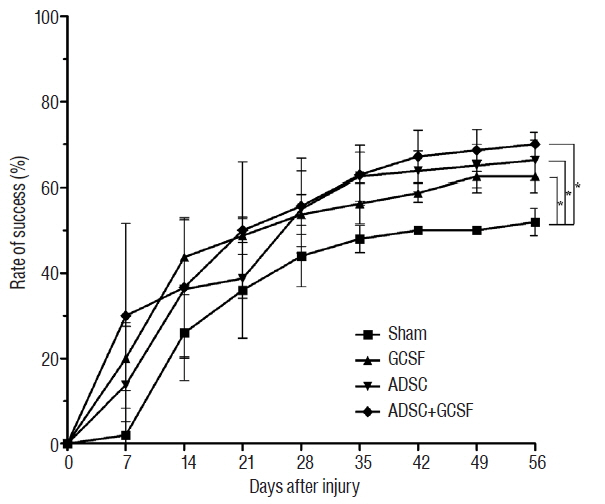
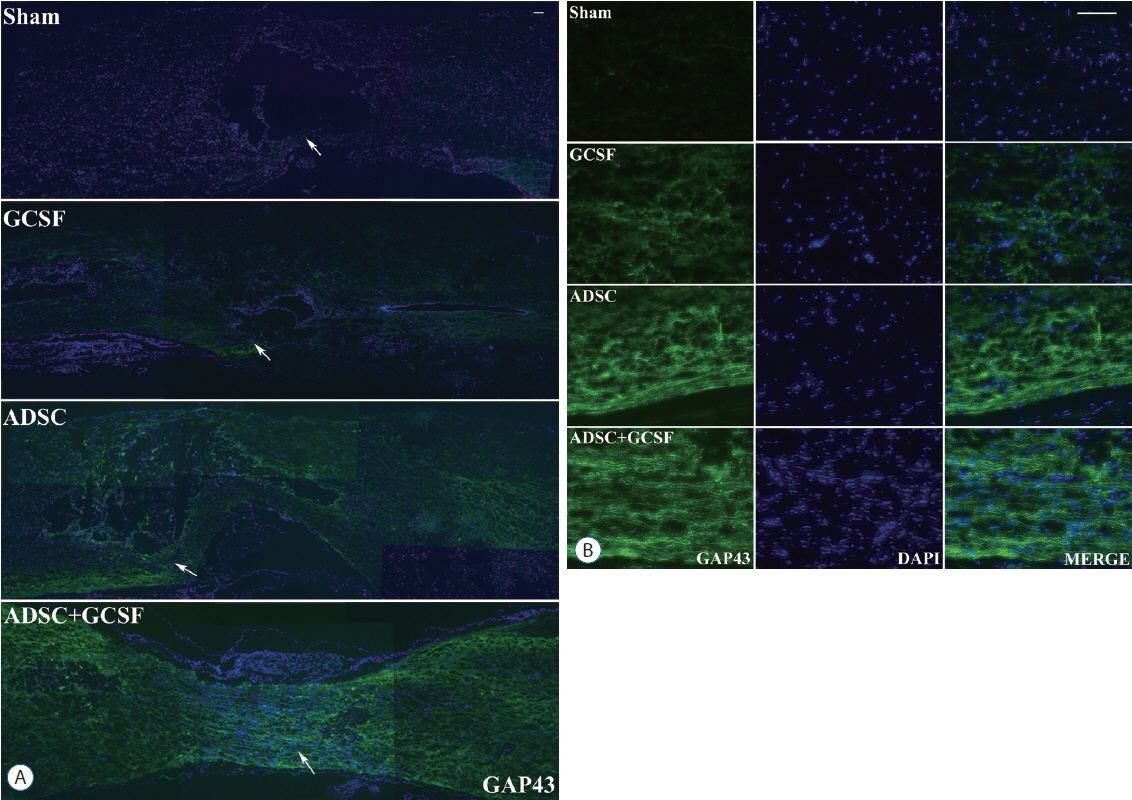
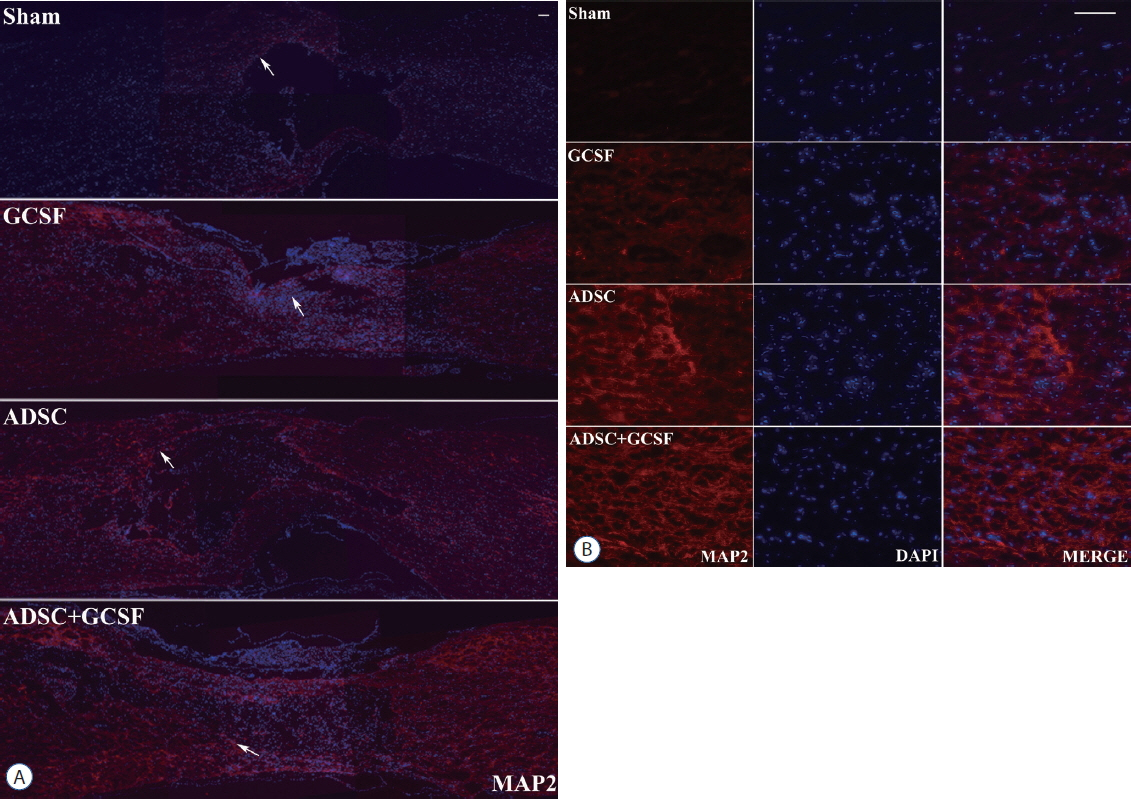
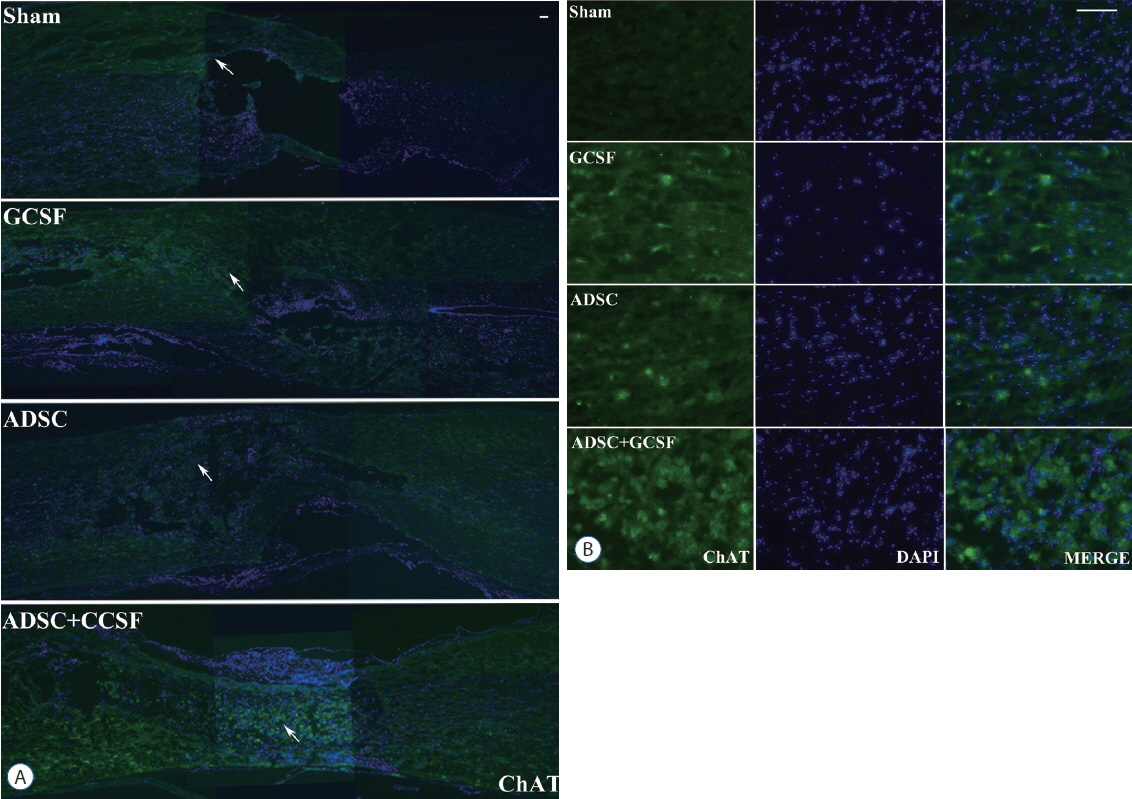
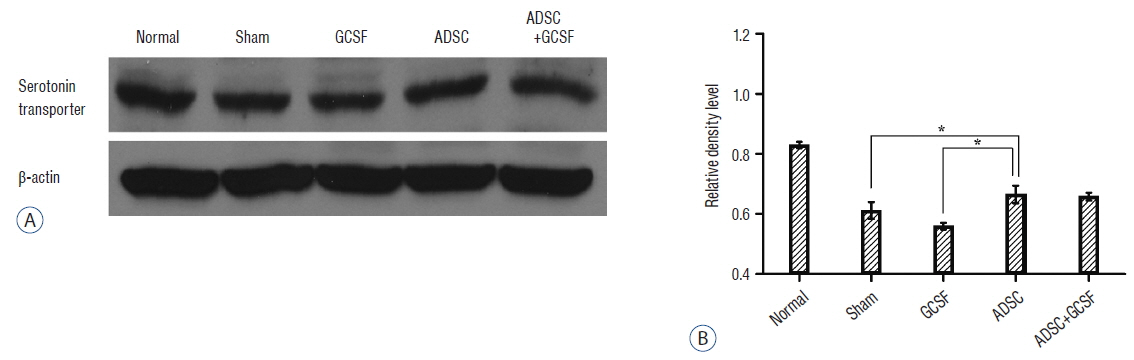
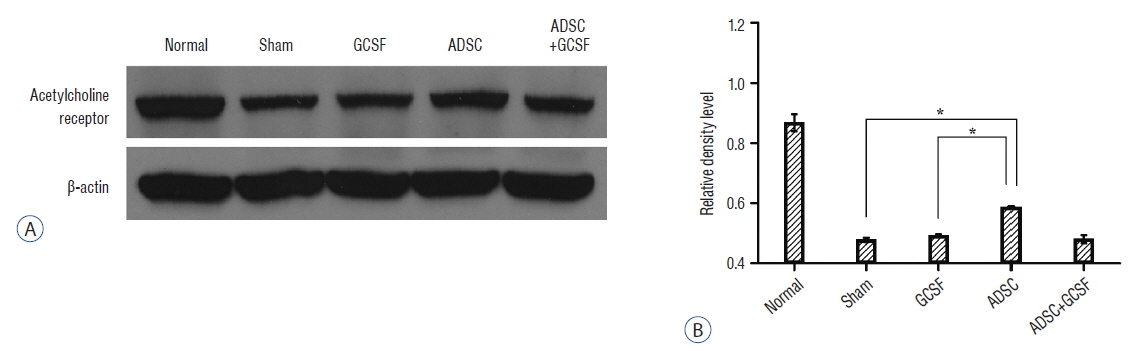
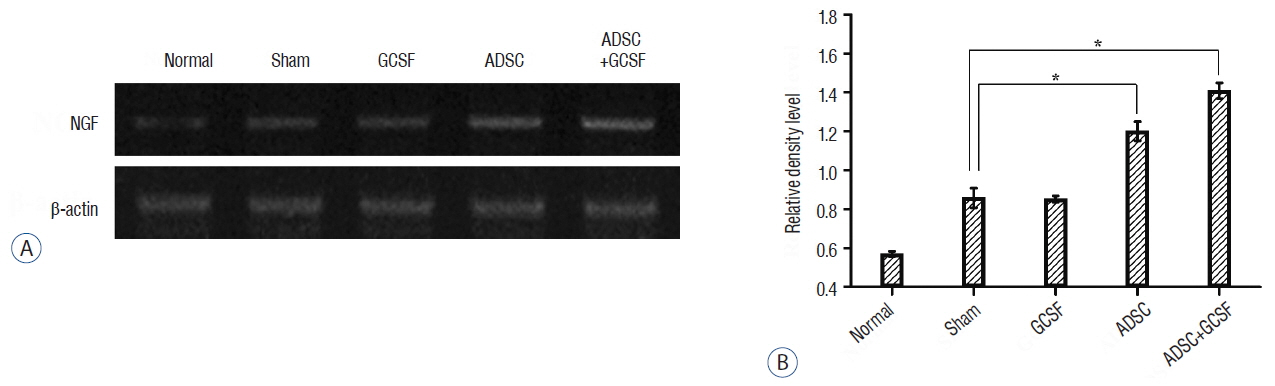
The ADSC and ADSC+GCSF groups, but not the GCSF group, showed significantly higher Basso-Beattie-Bresnahan scores than the Sham group during 8 weeks (p<0.01), but no significant difference between the ADSC and ADSC+GCSF groups. In the ladder rung test, all four groups were significantly different from each other, with the ADSC+GCSF group showing the best improvement (p<0.01). On immunofluorescent staining (GAP43, MAP2), western blotting (GAP43), and reverse transcription polymerase chain reaction (GAP43, nerve growth factor), the ADSC and ADSC+GCSF groups showed higher levels than the Sham and GCSF groups.
CONCLUSION
Our analyses suggest that the combination of GCSF and ADSCs infusions in acute SCI in the rat does not have a significant additive effect. Hence, when combination agents for SCI stem cell therapy are considered, molecules other than GCSF, or modifications to the methodology, should be investigated.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Combined Modality Therapy
Cytokines
GAP-43 Protein
Granulocyte Colony-Stimulating Factor
Mesenchymal Stromal Cells
Models, Animal*
Polymerase Chain Reaction
Rats*
Rats, Sprague-Dawley
Reverse Transcription
Spinal Cord Injuries*
Spinal Cord*
Stem Cells*
Cytokines
GAP-43 Protein
Granulocyte Colony-Stimulating Factor
Figure
Cited by 1 articles
-
Current Status and Future Strategies to Treat Spinal Cord Injury with Adult Stem Cells
Seong Kyun Jeong, Il Choi, Sang Ryong Jeon
J Korean Neurosurg Soc. 2020;63(2):153-162. doi: 10.3340/jkns.2019.0146.
Reference
-
References
1. Aguilar RM, Steward O. A bilateral cervical contusion injury model in mice: assessment of gripping strength as a measure of forelimb motor function. Exp Neurol. 221:38–53. 2010.
Article2. Barry FP. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today. 69:250–256. 2003.
Article3. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. 1995.
Article4. Beveridge RA, Miller JA, Kales AN, Binder RA, Robert NJ, Harvey JH, et al. A comparison of efficacy of sargramostim (yeast-derived RhuGM-CSF) and filgrastim (bacteria-derived RhuG-CSF) in the therapeutic setting of chemotherapy-induced myelosuppression. Cancer Invest. 16:366–373. 1998.
Article5. Choi JS, Leem JW, Lee KH, Kim SS, Suh-Kim H, Jung SJ, et al. Effects of human mesenchymal stem cell transplantation combined with polymer on functional recovery following spinal cord hemisection in rats. Korean J Physiol Pharmacol. 16:405–411. 2012.
Article6. Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol. 26:1167–1180. 2006.
Article7. Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G, et al. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol. 208:282–288. 2006.
Article8. Couto PA, Filipe VM, Magalhaes LG, Pereira JE, Costa LM, Melo-Pinto P, et al. A comparison of two-dimensional and three-dimensional techniques for the determination of hindlimb kinematics during treadmill locomotion in rats following spinal cord injury. J Neurosci Methods. 173:193–200. 2008.
Article9. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 78:2791–2808. 1991.
Article10. Deng YB, Yuan QT, Liu XG, Liu XL, Liu Y, Liu ZG, et al. Functional recovery after rhesus monkey spinal cord injury by transplantation of bone marrow mesenchymal-stem cell-derived neurons. Chin Med J (Engl). 118:1533–1541. 2005.11. Dittgen T, Pitzer C, Plaas C, Kirsch F, Vogt G, Laage R, et al. Granulocyte-colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS One. 7:e29880. 2012.
Article12. Furuya T, Hashimoto M, Koda M, Okawa A, Murata A, Takahashi K, et al. Treatment of rat spinal cord injury with a Rho-kinase inhibitor and bone marrow stromal cell transplantation. Brain Res. 1295:192–202. 2009.
Article13. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 58:137–160. 2003.
Article14. Hodgetts SI, Simmons PJ, Plant GW. A comparison of the behavioral and anatomical outcomes in sub-acute and chronic spinal cord injury models following treatment with human mesenchymal precursor cell transplantation and recombinant decorin. Exp Neurol. 248:343–359. 2013.
Article15. Jeon SR, Park JH, Lee JH, Kim DY, Kim HS, Sung IY, et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng Regen Med. 7:316–322. 2010.16. Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir (Wien). 152:1771–1777. 2010.
Article17. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 23:412–423. 2005.
Article18. Kawabe J, Koda M, Hashimoto M, Fujiyoshi T, Furuya T, Endo T, et al. Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats: laboratory investigation. J Neurosurg Spine. 15:414–421. 2011.
Article19. Kim KN, Oh SH, Lee KH, Yoon DH. Effect of human mesenchymal stem cell transplantation combined with growth factor infusion in the repair of injured spinal cord. Acta Neurochir Suppl. 99:133–136. 2006.
Article20. Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 24:702–708. 2010.
Article21. Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2:e156. 2007.
Article22. Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, et al. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 67:13–22. 2007.23. Liang P, Jin LH, Liang T, Liu EZ, Zhao SG. Human neural stem cells promote corticospinal axons regeneration and synapse reformation in injured spinal cord of rats. Chin Med J (Engl). 119:1331–1338. 2006.
Article24. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, et al. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 8:275–282. 2007.
Article25. Mukaetova-Ladinska EB, Andras A, Milne J, Abdel-All Z, Borr I, Jaros E, et al. Synaptic proteins and choline acetyltransferase loss in visual cortex in dementia with Lewy bodies. J Neuropathol Exp Neurol. 72:53–60. 2013.
Article26. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48:3464–3474. 2003.
Article27. Nandoe Tewarie RD, Hurtado A, Ritfeld GJ, Rahiem ST, Wendell DF, Barroso MM, et al. Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J Neurotrauma. 26:2313–2322. 2009.
Article28. Nishi RA, Liu H, Chu Y, Hamamura M, Su MY, Nalcioglu O, et al. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J Neurotrauma. 24:674–689. 2007.
Article29. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 11:897–911. 2009.
Article30. Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 70:1238–1247. discussion 1247. 2012.
Article31. Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 24:835–845. 2007.
Article32. Pitzer C, Klussmann S, Kruger C, Letellier E, Plaas C, Dittgen T, et al. The hematopoietic factor granulocyte-colony stimulating factor improves outcome in experimental spinal cord injury. J Neurochem. 113:930–942. 2010.
Article33. Quertainmont R, Cantinieaux D, Botman O, Sid S, Schoenen J, Franzen R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 7:e39500. 2012.
Article34. Ritfeld GJ, Nandoe Tewarie RD, Vajn K, Rahiem ST, Hurtado A, Wendell DF, et al. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 21:1561–1575. 2012.
Article35. Rodbell M. Metabolism of isolated fat cells. I Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 239:375–380. 1964.36. Roussos I, Rodriguez M, Villan D, Ariza A, Rodriguez L, Garcia J. Development of a rat model of spinal cord injury and cellular transplantation. Transplant Proc. 37:4127–4130. 2005.
Article37. Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J Biol Chem. 278:47585–47593. 2003.
Article38. Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 25:818–827. 2007.
Article39. Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 20:179–193. 2003.
Article40. Shin DA, Kim JM, Kim HI, Yi S, Ha Y, Yoon DH, et al. Comparison of functional and histological outcomes after intralesional, intracisternal, and intravenous transplantation of human bone marrow-derived mesenchymal stromal cells in a rat model of spinal cord injury. Acta Neurochir (Wien). 155:1943–1950. 2013.
Article41. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 110:1847–1854. 2004.
Article42. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 24:1217–1281. 2001.
Article43. Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien). 153:1003–1010. 2011.
Article44. Sykova E, Jendelova P, Urdzikova L, Lesny P, Hejcl A. Bone marrow stem cells and polymer hydrogels-two strategies for spinal cord injury repair. Cell Mol Neurobiol. 26:1113–1129. 2006.
Article45. Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 7:628–643. 2006.
Article46. Tobias CA, Han SS, Shumsky JS, Kim D, Tumolo M, Dhoot NO, et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 22:138–156. 2005.
Article47. Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 23:1379–1391. 2006.
Article48. Weaver CH, Buckner CD, Longin K, Appelbaum FR, Rowley S, Lilleby K, et al. Syngeneic transplantation with peripheral blood mononuclear cells collected after the administration of recombinant human granulocyte colony-stimulating factor. Blood. 82:1981–1984. 1993.
Article49. Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE. Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 29:169–178. 2011.
Article50. Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, et al. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 15:434–448. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs
- Transplantation of Human Adipose-derived Stromal Cells Promotes Functional Recovery of Rat Spinal Cord Injury
- Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver
- The Effect of Human Adipose Tissue Derived Mesenchymal Stem Cells and Growth Hormone on the Recovery of Neurological Deficits due to Experimental Spinal Cord Injury in Rat