Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ohkweon@snu.ac.kr
- 2Laboratory of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr
- 3College of Veterinary Medicine, Research Institute of Veterinary Medicine, Chungnam National University, Daejeon 305-764, Korea.
- KMID: 1726898
- DOI: http://doi.org/10.4142/jvs.2009.10.4.273
Abstract
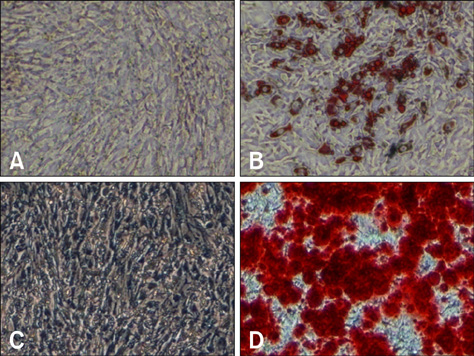
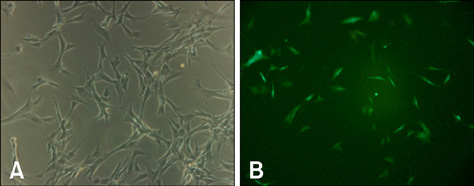
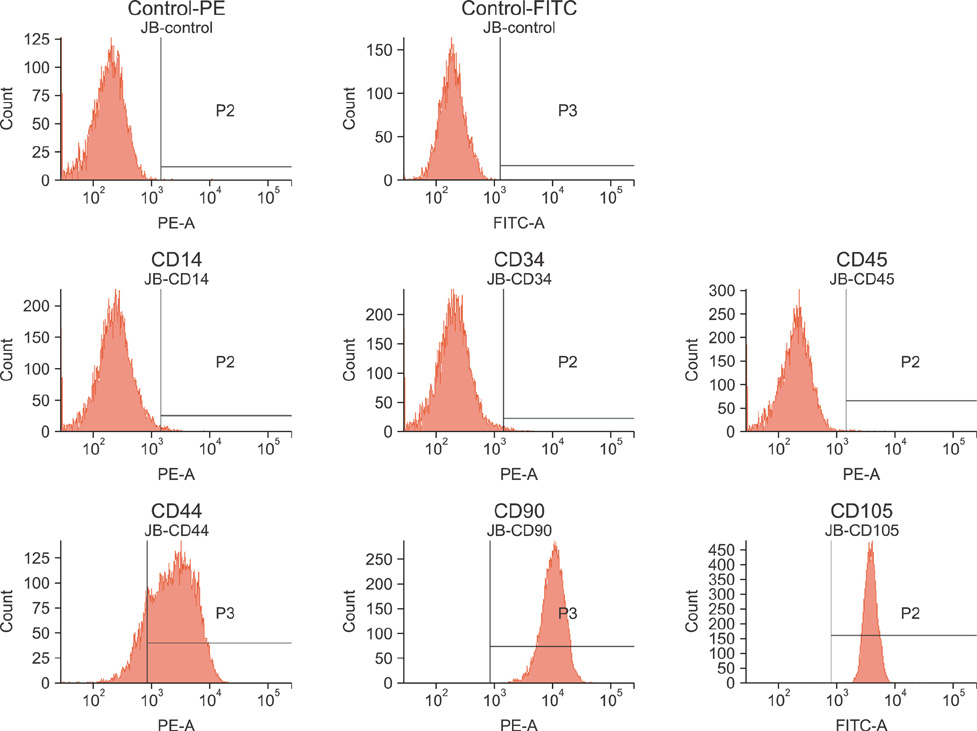
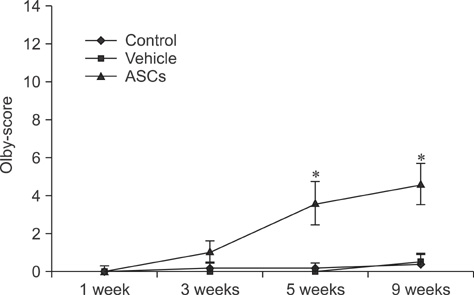
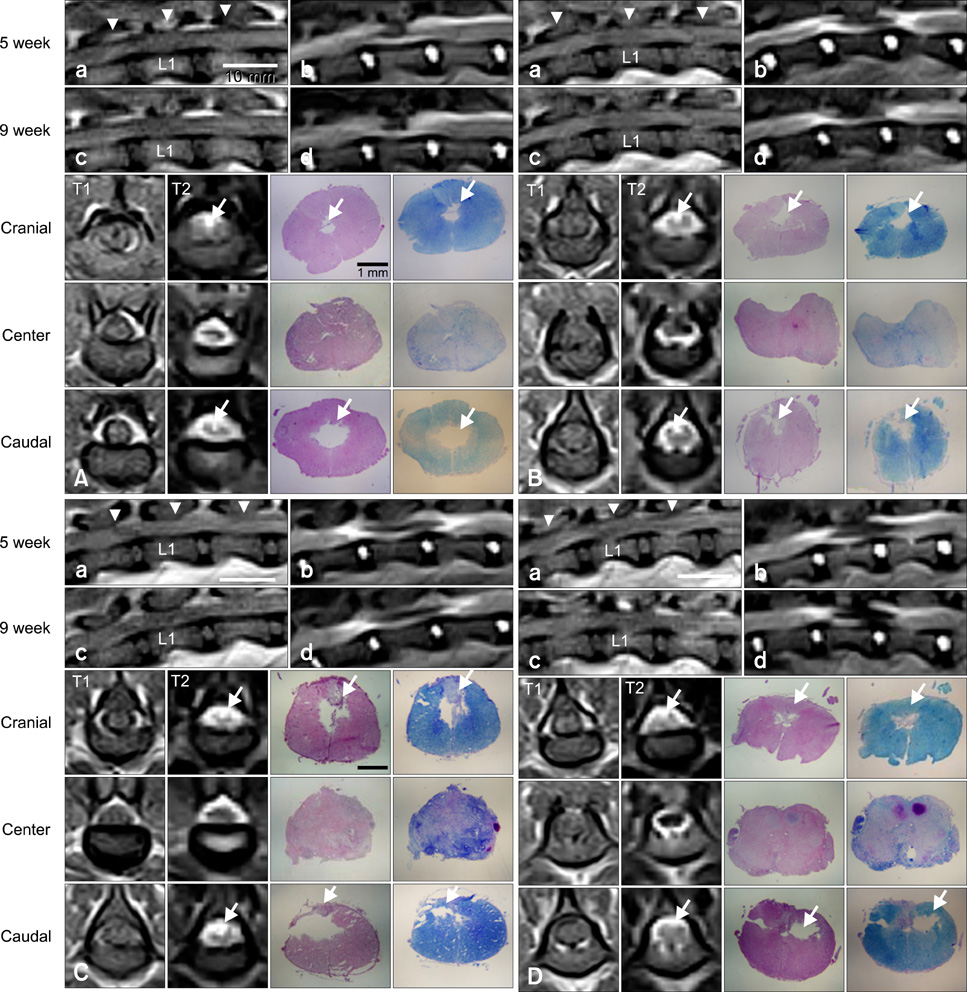
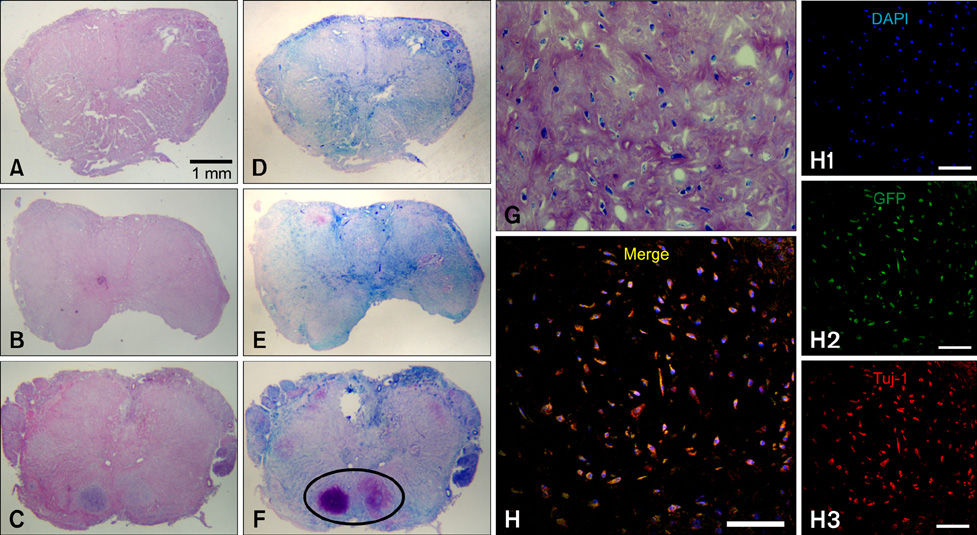
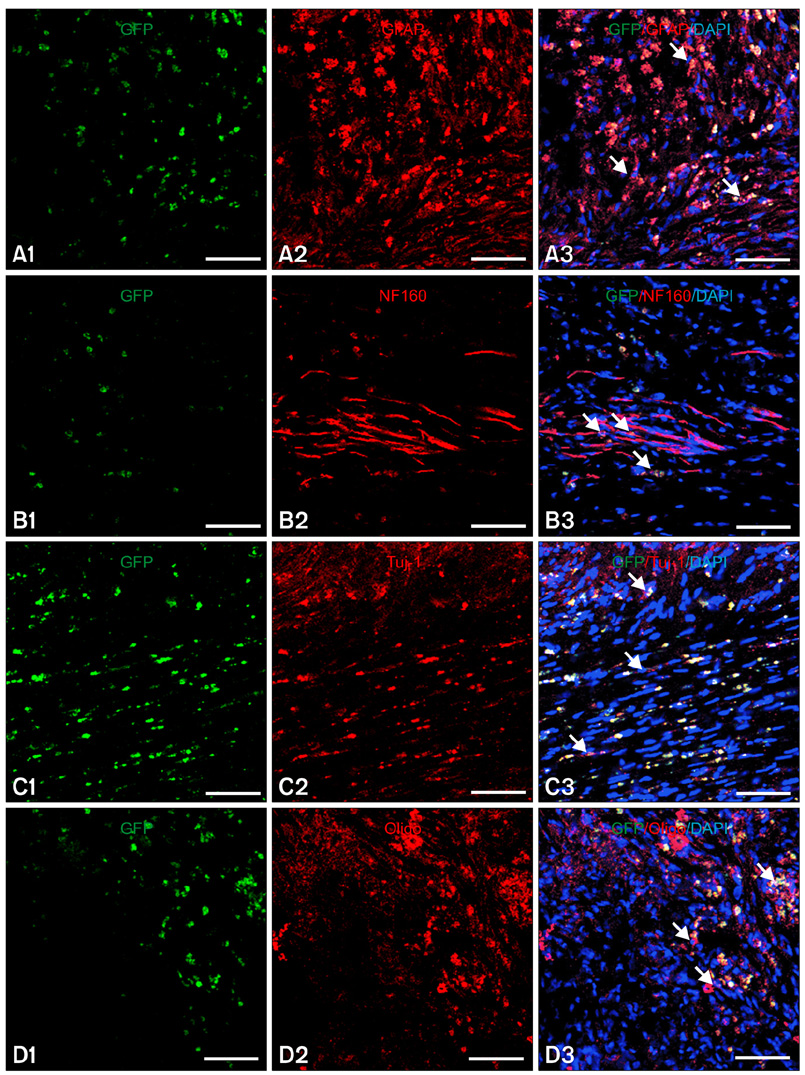
- In this study, we evaluated if the implantation of allogenic adipose-derived stem cells (ASCs) improved neurological function in a canine spinal cord injury model. Eleven adult dogs were assigned to three groups according to treatment after spinal cord injury by epidural balloon compression: C group (no ASCs treatment as control), V group (vehicle treatment with PBS), and ASC group (ASCs treatment). ASCs or vehicle were injected directly into the injured site 1 week after spinal cord injury. Pelvic limb function after transplantation was evaluated by Olby score. Magnetic resonance imaging, somatosensory evoked potential (SEP), histopathologic and immunohistichemical examinations were also performed. Olby scores in the ASC group increased from 2 weeks after transplantation and were significantly higher than C and V groups until 8 weeks (p<0.05). However, there were no significant differences between the C and V groups. Nerve conduction velocity based on SEP was significantly improved in the ASC group compared to C and V groups (p < 0.05). Positive areas for Luxol fast blue staining were located at the injured site in the ASC group. Also, GFAP, Tuj-1 and NF160 were observed immunohistochemically in cells derived from implanted ASCs. These results suggested that improvement in neurological function by the transplantation of ASCs in dogs with spinal cord injury may be partially due to the neural differentiation of implanted stem cells.
MeSH Terms
Figure
Cited by 7 articles
-
Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
Daeyoung Yoon, Byung-Jae Kang, Yongsun Kim, Seung Hoon Lee, Daeun Rhew, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2015;16(4):397-404. doi: 10.4142/jvs.2015.16.4.397.Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs
Yongsun Kim, Seung Hoon Lee, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2016;17(1):123-126. doi: 10.4142/jvs.2016.17.1.123.Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood
Hyo-Jong Lee, Kyung-Sun Kang, Sun-Young Kang, Hyung-Sik Kim, Se-Jin Park, Seung-Yong Lee, Kwang-Dong Kim, Hee-Chun Lee, Ji-Kwon Park, Won-Young Paik, Lyon Lee, Seong-Chan Yeon
J Vet Sci. 2016;17(3):289-297. doi: 10.4142/jvs.2016.17.3.289.Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury
Seung Hoon Lee, Yongsun Kim, Daeun Rhew, Ahyoung Kim, Kwang Rae Jo, Yongseok Yoon, Kyeung Uk Choi, Taeseong Jung, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2017;18(3):377-386. doi: 10.4142/jvs.2017.18.3.377.Use of stem-cell sheets expressing bone morphogenetic protein-7 in the management of a nonunion radial fracture in a Toy Poodle
Jaeyong Song, Yongsun Kim, Oh-Kyeong Kweon, Byung-Jae Kang
J Vet Sci. 2017;18(4):555-558. doi: 10.4142/jvs.2017.18.4.555.Frozen-thawed gelatin-induced osteogenic cell sheets of canine adipose-derived mesenchymal stromal cells improved fracture healing in canine model
Yongseok Yoon, Taeseong Jung, Muhammad Afan Shahid, Imdad Ullah Khan, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2019;20(6):. doi: 10.4142/jvs.2019.20.e63.Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review
Sun Kyu Oh, Sang Ryong Jeon
Korean J Neurotrauma. 2016;12(2):40-46. doi: 10.13004/kjnt.2016.12.2.40.
Reference
-
1. Anderson TE. A controlled pneumatic technique for experimental spinal cord contusion. J Neurosci Methods. 1982. 6:327–333.
Article2. Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair--what can it achieve? Nat Clin Pract Neurol. 2007. 3:152–161.
Article3. Berens SA, Colvin DC, Yu CG, Yezierski RP, Mareci TH. Evaluation of the pathologic characteristics of excitotoxic spinal cord injury with MR imaging. AJNR Am J Neuroradiol. 2005. 26:1612–1622.4. Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005. 16:1131–1141.
Article5. Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of Function after Spinal Cord Injury: Mechanisms Underlying Transplant-Mediated Recovery of Function Differ after Spinal Cord Injury in Newborn and Adult Rats. Exp Neurol. 1993. 123:3–16.
Article6. Cova L, Ratti A, Volta M, Fogh I, Cardin V, Corbo M, Silani V. Stem cell therapy for neurodegenerative diseases: the issue of transdifferentiation. Stem Cells Dev. 2004. 13:121–131.
Article7. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004. 22:560–567.
Article8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.
Article9. Duncan EG, Lemaire C, Armstrong RL, Tator CH, Potts DG, Linden RD. High-resolution magnetic resonance imaging of experimental spinal cord injury in the rat. Neurosurgery. 1992. 31:510–519.
Article10. Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005. 14:171–180.
Article11. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003. 5:362–369.
Article12. Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006. 498:525–538.
Article13. Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004. 6:543–553.
Article14. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.
Article15. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003. 182:399–411.
Article16. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008. 17:681–693.
Article17. Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003. 183:355–366.
Article18. Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006. 15:583–594.
Article19. Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979. 51:841–845.20. Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien). 2005. 147:985–992.
Article21. Lee JM. Evaluation of spinal cord dysfunction by the somatosensory evoked potentials (SEPs) in dogs [Ph.D dissertation]. 2000. Seoul: Seoul National University.22. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007. 8:275–282.
Article23. Ma WQ, Zhang SC, Li M, Yan YB, Ni CR. Experimental study of peripheral nerve grafts for repairing of chronic spinal cord injury in adult rats. Zhongguo Gu Shang. 2008. 21:519–521.24. Mihai G, Nout YS, Tovar CA, Miller BA, Schmalbrock P, Bresnahan JC, Beattie MS. Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J Neurotrauma. 2008. 25:1–18.
Article25. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006. 24:376–385.
Article26. Muir WW 3rd, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res. 2003. 64:1155–1160.
Article27. Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006. 1:13–20.
Article28. Olby NJ, De Risio L, Munana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001. 62:1624–1628.
Article29. Poncelet L, Michaux C, Balligand M. Study of spinal cord evoked injury potential by use of computer modeling and in dogs with naturally acquired thoracolumbar spinal cord compression. Am J Vet Res. 1998. 59:300–306.30. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.
Article31. Rucker NC, Lumb WV, Scott RJ. Combined pharmacologic and surgical treatments for acute spinal cord trauma. Am J Vet Res. 1981. 42:1138–1142.
Article32. Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005. 6:57–62.
Article33. Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004. 29:1971–1979.
Article34. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004. 5:146–156.
Article35. Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002. 5:438–445.
Article36. Vanický I, Urdzíkova L, Saganová K, Cízková D, Gálik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001. 18:1399–1407.
Article37. Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004. 155:225–230.
Article38. Weber T, Vroemen M, Behr V, Neuberger T, Jakob P, Haase A, Schuierer G, Bogdahn U, Faber C, Weidner N. In vivo high-resolution MR imaging of neuropathologic changes in the injured rat spinal cord. AJNR Am J Neuroradiol. 2006. 27:598–604.39. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article40. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS ONE. 2008. 3:e3336.
Article41. Yang JW, Jeong SM, Seo KM, Nam TC. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003. 4:97–101.
Article42. Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005. 50:247–257.
Article43. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002. 174:11–20.
Article44. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of Human Adipose-derived Stromal Cells Promotes Functional Recovery of Rat Spinal Cord Injury
- Fate of Transplanted Bone Marrow Derived Mesenchymal Stem Cells Following Spinal Cord Injury in Rats by Transplantation Routes
- Transplantation of Neural Stem Cells Cultured in Alginate Scaffold for Spinal Cord Injury in Rats
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- A Narrative Review of Advances in Neural Precursor Cell Transplantation Therapies for Spinal Cord Injury