Int J Stem Cells.
2017 May;10(1):1-11. 10.15283/ijsc17024.
From Bench to Market: Preparing Human Pluripotent Stem Cells Derived Cardiomyocytes for Various Applications
- Affiliations
-
- 1Department of Medicine, School of Medicine, Konkuk University, Seoul, Korea.
- 2Mirae Cell Bio Inc., Seoul, Korea.
- 3Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea. stemchung@gmail.com
- KMID: 2384596
- DOI: http://doi.org/10.15283/ijsc17024
Abstract
- Human cardiomyocytes (CMs) cease to proliferate and remain terminally differentiated thereafter, when humans reach the mid-20s. Thus, any damages sustained by myocardium tissue are irreversible, and they require medical interventions to regain functionality. To date, new surgical procedures and drugs have been developed, albeit with limited success, to treat various heart diseases including myocardial infarction. Hence, there is a pressing need to develop more effective treatment methods to address the increasing mortality rate of the heart diseases. Functional CMs are not only an important in vitro cellular tool to model various types of heart diseases for drug development, but they are also a promising therapeutic agent for cell therapy. However, the limited proliferative capacity entails difficulties in acquiring functional CMs in the scale that is required for pathological studies and cell therapy development. Stem cells, human pluripotent stem cells (hPSCs) in particular, have been considered as an unlimited cellular source for providing functional CMs for various applications. Notable progress has already been made: the first clinical trials of hPSCs derived CMs (hPSC-CMs) for treating myocardial infarction was approved in 2015, and their potential use in disease modeling and drug discovery is being fully explored. This concise review gives an account of current development of differentiation, purification and maturation techniques for hPSC-CMs, and their application in cell therapy development and pharmaceutical industries will be discussed with the latest experimental evidence.
MeSH Terms
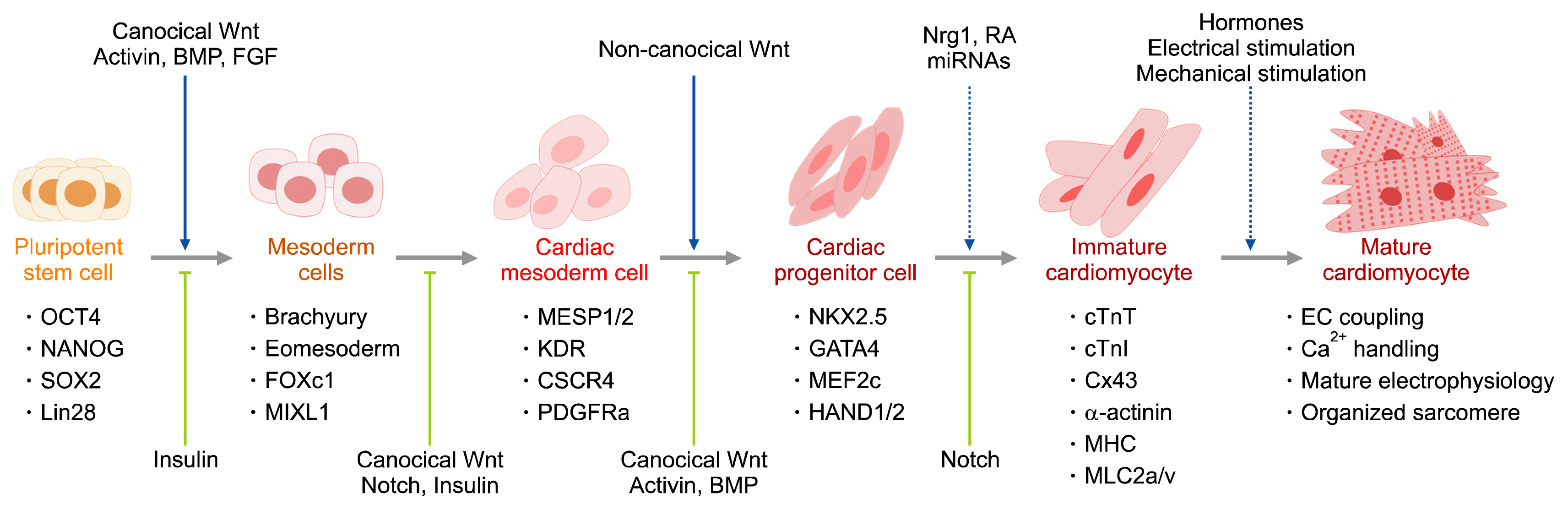
Figure
Reference
-
References
1. Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009; 104:e30–e41. DOI: 10.1161/CIRCRESAHA.108.192237. PMID: 19213953. PMCID: 2741334.
Article2. Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008; 26:2300–2310. DOI: 10.1634/stemcells.2008-0183. PMID: 18583540.
Article3. Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012; 485:599–604. DOI: 10.1038/nature11139. PMID: 22660318. PMCID: 3367390.
Article4. Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012; 111:1125–1136. DOI: 10.1161/CIRCRESAHA.112.273144. PMID: 22912385. PMCID: 3482164.
Article5. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014; 11:855–860. DOI: 10.1038/nmeth.2999. PMID: 24930130. PMCID: 4169698.
Article6. Lian X, Bao X, Zilberter M, Westman M, Fisahn A, Hsiao C, Hazeltine LB, Dunn KK, Kamp TJ, Palecek SP. Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods. 2015; 12:595–596. DOI: 10.1038/nmeth.3448. PMID: 26125590. PMCID: 4663075.
Article7. Mummery CL, Ward D, Passier R. Differentiation of human embryonic stem cells to cardiomyocytes by coculture with endoderm in serum-free medium. Curr Protoc Stem Cell Biol. 2007; DOI: 10.1002/9780470151808.sc01f02s2.
Article8. Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002; 91:659–661. DOI: 10.1161/01.RES.0000039084.30342.9B. PMID: 12386141.
Article9. Kempf H, Olmer R, Kropp C, Rückert M, Jara-Avaca M, Robles-Diaz D, Franke A, Elliott DA, Wojciechowski D, Fischer M, Roa Lara A, Kensah G, Gruh I, Haverich A, Martin U, Zweigerdt R. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports. 2014; 3:1132–1146. DOI: 10.1016/j.stemcr.2014.09.017. PMID: 25454631. PMCID: 4264033.
Article10. Doevendans PA, Kubalak SW, An RH, Becker DK, Chien KR, Kass RS. Differentiation of cardiomyocytes in floating embryoid bodies is comparable to fetal cardiomyocytes. J Mol Cell Cardiol. 2000; 32:839–851. DOI: 10.1006/jmcc.2000.1128. PMID: 10775488.
Article11. Xu C, Police S, Hassanipour M, Gold JD. Cardiac bodies: a novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev. 2006; 15:631–639. DOI: 10.1089/scd.2006.15.631. PMID: 17105398.
Article12. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007; 25:1015–1024. DOI: 10.1038/nbt1327. PMID: 17721512.
Article13. Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008; 453:524–528. DOI: 10.1038/nature06894. PMID: 18432194.
Article14. Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011; 8:228–240. DOI: 10.1016/j.stem.2010.12.008. PMID: 21295278.
Article15. Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011; 29:1011–1018. DOI: 10.1038/nbt.2005. PMID: 22020386. PMCID: 4949030.
Article16. Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011; 6:e23657. DOI: 10.1371/journal.pone.0023657. PMID: 21876760. PMCID: 3158088.
Article17. Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007; 15:2027–2036. DOI: 10.1038/sj.mt.6300303. PMID: 17895862.
Article18. Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, Skelton RJ, Ward-van Oostwaard D, Lim SM, Khammy O, Li X, Hawes SM, Davis RP, Goulburn AL, Passier R, Prall OW, Haynes JM, Pouton CW, Kaye DM, Mummery CL, Elefanty AG, Stanley EG. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011; 8:1037–1040. DOI: 10.1038/nmeth.1740. PMID: 22020065.
Article19. Ritner C, Wong SS, King FW, Mihardja SS, Liszewski W, Erle DJ, Lee RJ, Bernstein HS. An engineered cardiac reporter cell line identifies human embryonic stem cell-derived myocardial precursors. PLoS One. 2011; 6:e16004. DOI: 10.1371/journal.pone.0016004. PMID: 21245908. PMCID: 3014940.
Article20. Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013; 12:127–137. DOI: 10.1016/j.stem.2012.09.013.
Article21. Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010; 107:776–786. DOI: 10.1161/CIRCRESAHA.110.223917. PMID: 20671236. PMCID: 2941561.
Article22. Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010; 48:379–386. DOI: 10.1016/j.yjmcc.2009.09.016.
Article23. Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997; 80:88–94. DOI: 10.1161/01.RES.80.1.88. PMID: 8978327.
Article24. Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994; 90:713–725. DOI: 10.1161/01.CIR.90.2.713. PMID: 8044940.
Article25. Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010; 56:130–140. DOI: 10.1097/FJC.0b013e3181e74a14. PMID: 20505524.
Article26. Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013; 22:1991–2002. DOI: 10.1089/scd.2012.0490. PMID: 23461462. PMCID: 3699903.
Article27. Heidi Au HT, Cui B, Chu ZE, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009; 9:564–575. DOI: 10.1039/B810034A. PMID: 19190792.
Article28. Zimmermann WH, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002; 90:223–230. DOI: 10.1161/hh0202.103644. PMID: 11834716.
Article29. Martherus RS, Vanherle SJ, Timmer ED, Zeijlemaker VA, Broers JL, Smeets HJ, Geraedts JP, Ayoubi TA. Electrical signals affect the cardiomyocyte transcriptome independently of contraction. Physiol Genomics. 2010; 42A:283–289. DOI: 10.1152/physiolgenomics.00182.2009. PMID: 20858713.
Article30. Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013; 494:105–110. DOI: 10.1038/nature11799. PMID: 23354045. PMCID: 3753229.
Article31. Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Mol Cell Cardiol. 1996; 28:1463–1477. DOI: 10.1006/jmcc.1996.0137. PMID: 8841934.
Article32. Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011; 471:230–234. DOI: 10.1038/nature09855. PMID: 21307850. PMCID: 3077925.
Article33. Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010; 465:808–812. DOI: 10.1038/nature09005. PMID: 20535210. PMCID: 2885001.
Article34. Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, Seyfarth M, Sinnecker D, Schömig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010; 363:1397–1409. DOI: 10.1056/NEJMoa0908679. PMID: 20660394.
Article35. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013; 12:101–113. DOI: 10.1016/j.stem.2012.10.010. PMID: 23290139. PMCID: 3638033.
Article36. Huang HP, Chen PH, Hwu WL, Chuang CY, Chien YH, Stone L, Chien CL, Li LT, Chiang SC, Chen HF, Ho HN, Chen CH, Kuo HC. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum Mol Genet. 2011; 20:4851–4864. DOI: 10.1093/hmg/ddr424. PMID: 21926084.
Article37. Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004; 22:1282–1289. DOI: 10.1038/nbt1014. PMID: 15448703.
Article38. Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014; 35:2798–2808. DOI: 10.1016/j.biomaterials.2013.12.052. PMID: 24424206.
Article39. Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, Wu JC. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol. 2014; 64:436–448. DOI: 10.1016/j.jacc.2014.04.056. PMID: 25082575. PMCID: 4134946.
Article40. Yeghiazarians Y, Gaur M, Zhang Y, Sievers RE, Ritner C, Prasad M, Boyle A, Bernstein HS. Myocardial improvement with human embryonic stem cell-derived cardiomyocytes enriched by p38MAPK inhibition. Cytotherapy. 2012; 14:223–231. DOI: 10.3109/14653249.2011.623690. PMCID: 3270940.
Article41. Luo J, Weaver MS, Cao B, Dennis JE, Van Biber B, Laflamme MA, Allen MD. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Transl Med. 2014; 3:734–744. DOI: 10.5966/sctm.2013-0189. PMID: 24736402. PMCID: 4039456.
Article42. Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012; 126(11 Suppl 1):S29–S37. DOI: 10.1161/CIRCULATIONAHA.111.084343. PMID: 22965990.
Article43. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014; 510:273–277. DOI: 10.1038/nature13233. PMID: 24776797. PMCID: 4154594.
Article44. Shiba Y, Filice D, Fernandes S, Minami E, Dupras SK, Biber BV, Trinh P, Hirota Y, Gold JD, Viswanathan M, Laflamme MA. Electrical Integration of Human Embryonic Stem Cell-Derived Cardiomyocytes in a Guinea Pig Chronic Infarct Model. J Cardiovasc Pharmacol Ther. 2014; 19:368–381. DOI: 10.1177/1074248413520344. PMID: 24516260. PMCID: 4127378.
Article45. Sugiura T, Hibino N, Breuer CK, Shinoka T. Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardiomyocytes promoted the regeneration of host cardiomyocytes in a rat model. J Cardiothorac Surg. 2016; 11:163. DOI: 10.1186/s13019-016-0559-z. PMID: 27906085. PMCID: 5131419.
Article46. Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015; 36:2011–2017. DOI: 10.1093/eurheartj/ehv189. PMID: 25990469.
Article47. Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K, Kwan J, Park KS, Choi D, Jang YS, Hong MK. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014; 29:23–31. DOI: 10.3346/jkms.2014.29.1.23. PMID: 24431901. PMCID: 3890472.
Article48. Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006; 355:1199–1209. DOI: 10.1056/NEJMoa055706. PMID: 16990383.
Article49. Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005; 111:2198–2202. DOI: 10.1161/01.CIR.0000163546.27639.AA. PMID: 15851598.
Article50. Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011; 471:225–229. DOI: 10.1038/nature09747. PMID: 21240260.
Article51. Csöbönyeiová M, Polák Š, Danišovič L. Toxicity testing and drug screening using iPSC-derived hepatocytes, cardiomyocytes, and neural cells. Can J Physiol Pharmacol. 2016; 94:687–694. DOI: 10.1139/cjpp-2015-0459. PMID: 27128322.
Article52. Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, Jean JC, Omari A, Sampson KJ, Kotton DN, Keller G, Kass RS. Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol. 2013; 141:61–72. DOI: 10.1085/jgp.201210899. PMID: 23277474. PMCID: 3536519.
Article53. Shinozawa T, Nakamura K, Shoji M, Morita M, Kimura M, Furukawa H, Ueda H, Shiramoto M, Matsuguma K, Kaji Y, Ikushima I, Yono M, Liou SY, Nagai H, Nakanishi A, Yamamoto K, Izumo S. Recapitulation of clinical individual susceptibility to drug-induced QT prolongation in healthy subjects using iPSC-derived cardiomyocytes. Stem Cell Reports. 2017; 8:226–234. DOI: 10.1016/j.stemcr.2016.12.014. PMID: 28111276. PMCID: 5312248.
Article54. Stillitano F, Hansen J, Kong CW, Karakikes I, Funck-Brentano C, Geng L, Scott S, Reynier S, Wu M, Valogne Y, Desseaux C, Salem JE, Jeziorowska D, Zahr N, Li R, Iyengar R, Hajjar RJ, Hulot JS. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. Elife. 2017; 6:DOI: 10.7554/eLife.19406. PMID: 28134617. PMCID: 5279943.
Article55. Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012; 4:180–191. DOI: 10.1002/emmm.201100194. PMCID: 3376852.
Article56. Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J Am Coll Cardiol. 2012; 60:990–1000. DOI: 10.1016/j.jacc.2012.02.066. PMID: 22749309.
Article57. Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012; 4:130ra47. DOI: 10.1126/scitranslmed.3003552. PMID: 22517884. PMCID: 3657516.
Article58. Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014; 20:616–623. DOI: 10.1038/nm.3545. PMID: 24813252. PMCID: 4172922.
Article59. Okata S, Yuasa S, Suzuki T, Ito S, Makita N, Yoshida T, Li M, Kurokawa J, Seki T, Egashira T, Aizawa Y, Kodaira M, Motoda C, Yozu G, Shimojima M, Hayashiji N, Hashimoto H, Kuroda Y, Tanaka A, Murata M, Aiba T, Shimizu W, Horie M, Kamiya K, Furukawa T, Fukuda K. Embryonic type Na+ channel β-subunit, SCN3B masks the disease phenotype of Brugada syndrome. Sci Rep. 2016; 6:34198. DOI: 10.1038/srep34198.
Article60. Veerman CC, Mengarelli I, Guan K, Stauske M, Barc J, Tan HL, Wilde AA, Verkerk AO, Bezzina CR. hiPSC-derived cardiomyocytes from Brugada Syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci Rep. 2016; 6:30967. DOI: 10.1038/srep30967. PMID: 27485484. PMCID: 4971529.
Article61. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, Del Álamo JC, Wu SM, Moslehi JJ, Mercola M, Wu JC. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017; 9:DOI: 10.1126/scitranslmed.aaf2584. PMCID: 5409837.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maturation of Stem Cell-Derived Cardiomyocytes: Foe in Translation Medicine
- Cardiac Regeneration with Human Pluripotent Stem Cell-Derived Cardiomyocytes
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells