Int J Stem Cells.
2020 Nov;13(3):432-438. 10.15283/ijsc20074.
Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells
- Affiliations
-
- 1Institute of Regenerative Medicine, LifeNet Health, Virginia Beach, VA, USA
- KMID: 2508917
- DOI: http://doi.org/10.15283/ijsc20074
Abstract
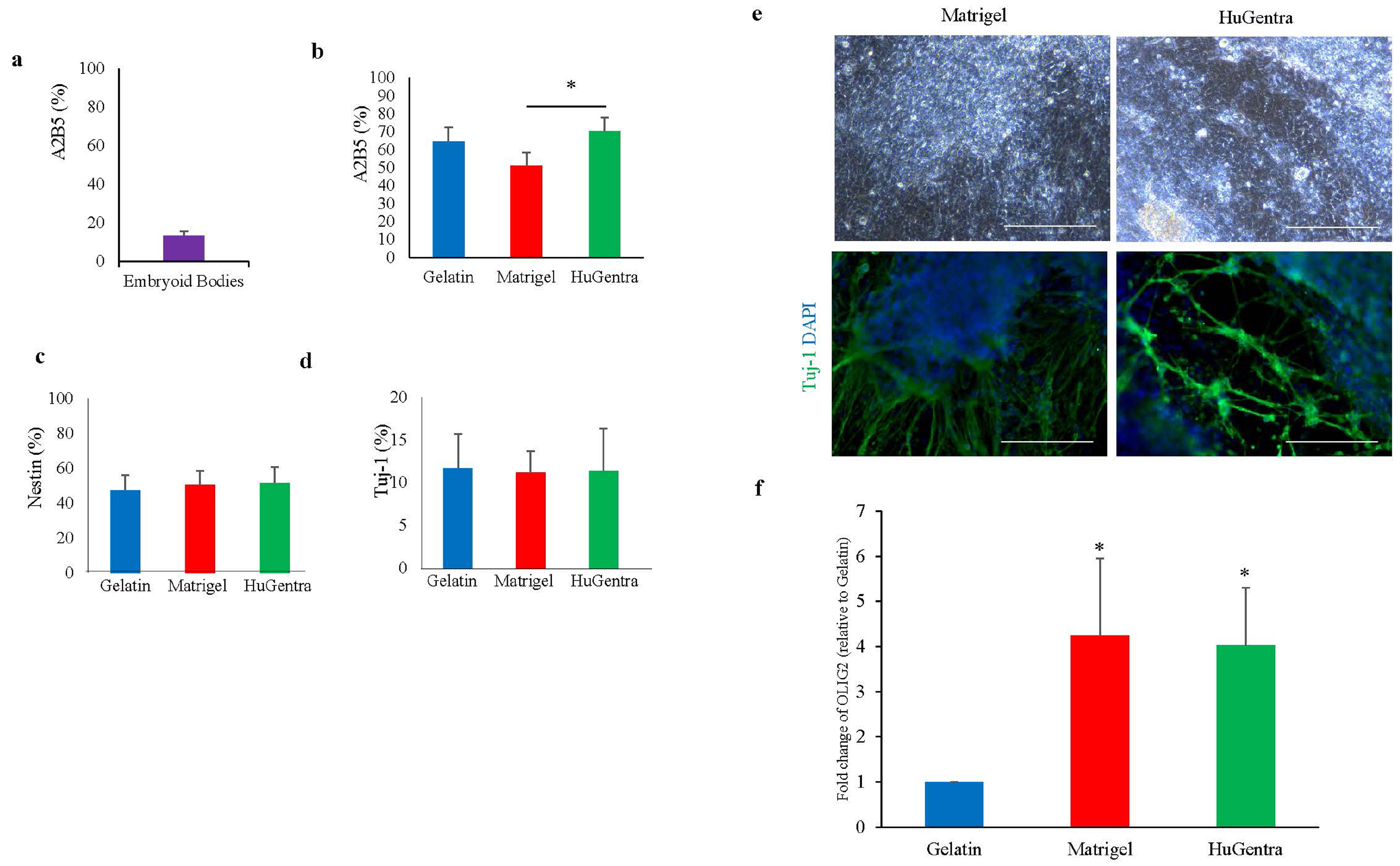
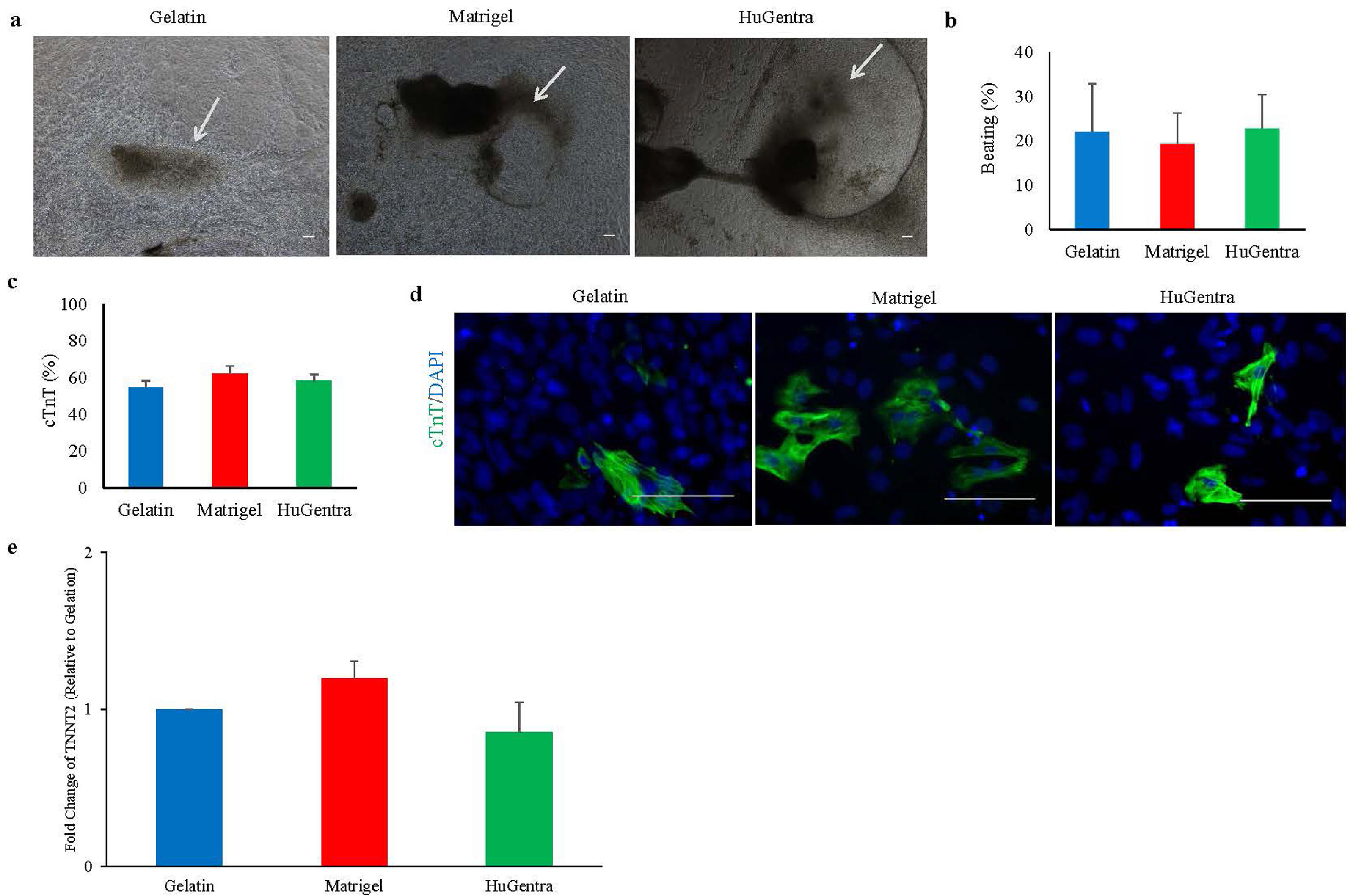
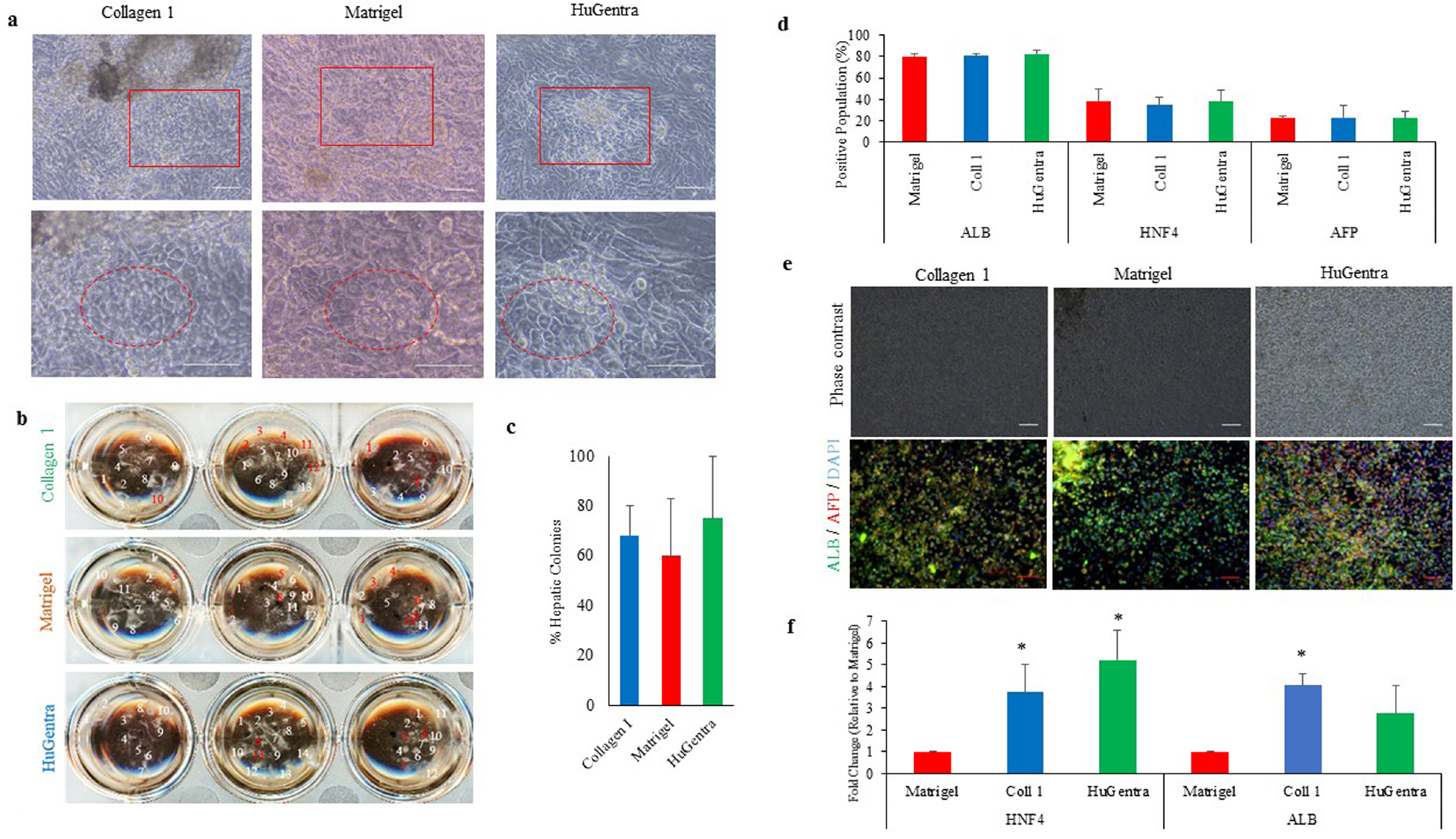
- Human pluripotent stem cells (hPSCs) hold great promise for future applications in drug discovery and cell therapies. hPSC culture protocols require specific substrates and medium supplements to support cell expansion and lineage specific differentiation. The animal origin of these substrates is a severe limitation when considering the translation of hPSC derivatives to the clinic and in vitro disease modeling. The present study evaluates the use of a human placenta-derived extracellular matrix (ECM) hydrogel, HuGentraⓇ , to support tri-lineage differentiation of human induced pluripotent stem cells (hiPSCs). Lineage-specific embryoid bodies (EBs) were plated onto three separate matrices, and differentiation efficiency was evaluated based on morphology, protein, and gene expression. HuGentra was found to support the differentiation of hiPSCs to all three germ layers: ectodermal, mesodermal, and endodermal lineages. hiPSCs differentiated into neurons, cardiomyocytes, and hepatocytes on HuGentra had similar morphology, protein, and gene expression compared to differentiation on Matrigel or other cell preferred matrices. HuGentra can be considered as a suitable human substrate for hiPSC differentiation.
Keyword
Figure
Reference
-
References
1. Kleinman HK, Martin GR. 2005; Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 15:378–386. DOI: 10.1016/j.semcancer.2005.05.004. PMID: 15975825.
Article2. Del Álamo JC, Lemons D, Serrano R, Savchenko A, Cerignoli F, Bodmer R, Mercola M. 2016; High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim Biophys Acta. 1863(7 Pt B):1717–1727. DOI: 10.1016/j.bbamcr.2016.03.003. PMID: 26952934. PMCID: PMC4885786.
Article3. Kondo Y, Iwao T, Nakamura K, Sasaki T, Takahashi S, Kamada N, Matsubara T, Gonzalez FJ, Akutsu H, Miyagawa Y, Okita H, Kiyokawa N, Toyoda M, Umezawa A, Nagata K, Matsunaga T, Ohmori S. 2014; An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab Pharmacokinet. 29:237–243. DOI: 10.2133/dmpk.DMPK-13-RG-104. PMID: 24334537. PMCID: PMC6594155.
Article4. Lee JB, Graham M, Collins TJ, Lee JH, Hong SH, Mcnicol AJ, Shapovalova Z, Bhatia M. 2015; Reversible lineage-specific priming of human embryonic stem cells can be exploited to optimize the yield of differentiated cells. Stem Cells. 33:1142–1152. DOI: 10.1002/stem.1952. PMID: 25639500. PMCID: PMC4413029.
Article5. Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. 2012; Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 109:E1848–E1857. DOI: 10.1073/pnas.1200250109. PMID: 22645348. PMCID: PMC3390875.
Article6. Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. 2012; Derivation of xeno-free and GMP-grade human embryonic stem cells--platforms for future clinical applications. PLoS One. 7:e35325. DOI: 10.1371/journal.pone.0035325. PMID: 22745653. PMCID: PMC3380026.7. Zhao C, Ikeya M. 2018; Generation and applications of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Int. 2018:9601623. DOI: 10.1155/2018/9601623. PMID: 30154868. PMCID: PMC6091255.
Article8. Hongisto H, Ilmarinen T, Vattulainen M, Mikhailova A, Skottman H. 2017; Xeno- and feeder-free differentiation of human pluripotent stem cells to two distinct ocular epithelial cell types using simple modifications of one method. Stem Cell Res Ther. 8:291. DOI: 10.1186/s13287-017-0738-4. PMID: 29284513. PMCID: PMC5747074.
Article9. Farzaneh Z, Pakzad M, Vosough M, Pournasr B, Baharvand H. 2014; Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem Cell Biol. 142:217–226. DOI: 10.1007/s00418-014-1183-4. PMID: 24477550.
Article10. Ullah I, Busch JF, Rabien A, Ergün B, Stamm C, Knosalla C, Hippenstiel S, Reinke P, Kurtz A. 2020; Adult tissue extracellular matrix determines tissue specification of human iPSC-derived embryonic stage mesodermal precursor cells. Adv Sci (Weinh). 7:1901198. DOI: 10.1002/advs.201901198. PMID: 32154066. PMCID: PMC7055561.
Article11. Francis MP, Breathwaite E, Bulysheva AA, Varghese F, Rodriguez RU, Dutta S, Semenov I, Ogle R, Huber A, Tichy AM, Chen S, Zemlin C. 2017; Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Bio-mater. 52:92–104. DOI: 10.1016/j.actbio.2016.12.027. PMID: 27965171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lymphoid Lineage γδ T Cells Were Successfully Generated from Human Pluripotent Stem Cells via Hemogenic Endothelium
- Do the Fibroblasts Contained in Early Passage MSC Population Adversely Affect the Characteristics of Stem Cell Population Obtained from Human Placenta?
- Neuronal Differentiation of a Human Induced Pluripotent Stem Cell Line (FS-1) Derived from Newborn Foreskin Fibroblasts
- Transcriptional Profiles of Imprinted Genes in Human Embryonic Stem Cells During In vitro Differentiation
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation