Immune Netw.
2017 Jun;17(3):171-178. 10.4110/in.2017.17.3.171.
Effects of Cellular 11β-hydroxysteroid Dehydrogenase 1 on LPS-induced Inflammatory Responses in Synovial Cell Line, SW982
- Affiliations
-
- 1Department of Pharmacy, Keimyung University, Daegu 42601, Korea. yscho123@kmu.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Mokpo National University, Mokpo 58554, Korea.
- KMID: 2383355
- DOI: http://doi.org/10.4110/in.2017.17.3.171
Abstract
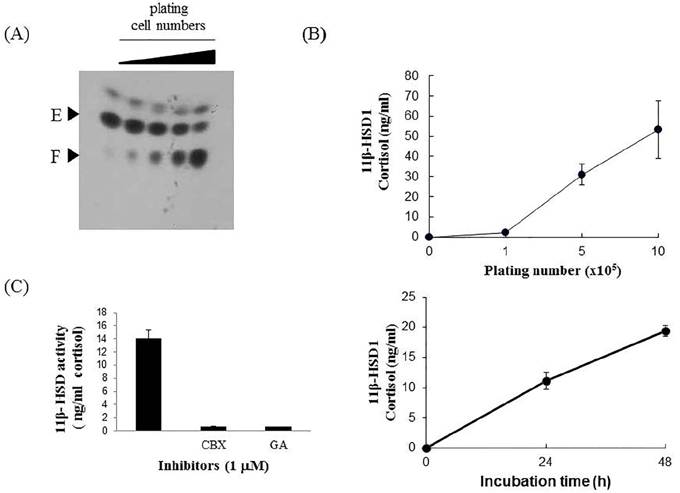
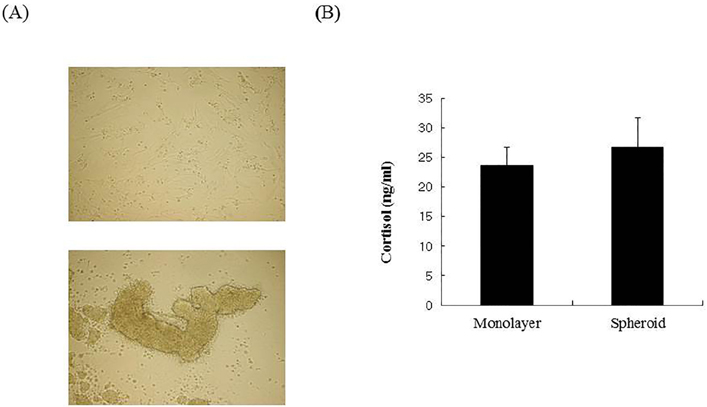
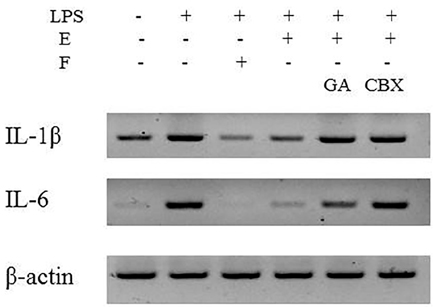
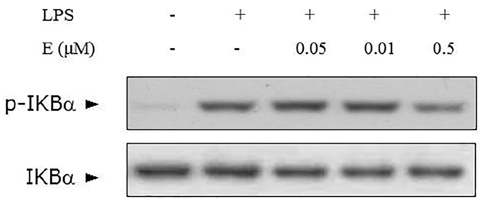
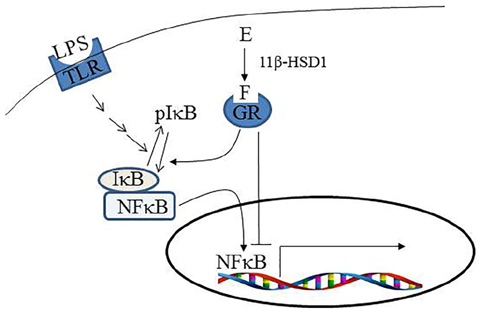
- 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) catalyzes the conversion of inactive cortisone into active cortisol, which has pleiotropic roles in various biological conditions, such as immunological and metabolic homeostasis. Cortisol is mainly produced in the adrenal gland, but can be locally regenerated in the liver, fat, and muscle. Its diverse actions are primarily mediated by binding to the glucocorticoid receptor. SW982, a human synovial cell line, expresses 11β-HSD type 1, but not type 2, that catalyzes the conversion of cortisone to cortisol. In this study, therefore, we investigated the control of lipopolysaccharide (LPS)-induced inflammatory responses by prereceptor regulation-mediated maintenance of cortisol levels. Preliminarily, cell seeding density and incubation period were optimized for analyzing the catalytic activity of SW982. Additionally, cellular 11β-HSD1 still remained active irrespective of monolayer or spheroid culture conditions. Inflammatory stimulants, such as interleukin (IL)-1β, tumor necrosis factor (TNF)α, and LPS, did not affect the catalytic activity of 11β-HSD1, although a high dose of LPS significantly decreased its activity. Additionally, autocrine effects of cortisol on inflammatory responses were investigated in LPS-stimulated SW982 cells. LPS upregulated pro-inflammatory cytokines, including IL-6 and IL-1β, in SW982 cells, while cortisol production, catalyzed by cellular 11β-HSD1, downregulated LPS-stimulated cytokines. Furthermore, suppression of NFκB activation-mediated pro-inflammatory responses by cortisol was revealed. In conclusion, the activity of cellular 11β-HSD1 was closely correlated with suppression of LPS-induced inflammation. Therefore, these results partly support the notion that prereceptor regulation of locally regenerated cortisol could be taken into consideration for treatment of inflammation-associated diseases, including arthritis.
MeSH Terms
-
Adrenal Glands
Arthritis
Cell Line*
Cortisone
Cytokines
Homeostasis
Humans
Hydrocortisone
Inflammation
Interleukin-6
Interleukins
Liver
Oxidoreductases*
Receptors, Glucocorticoid
Tumor Necrosis Factor-alpha
Cortisone
Cytokines
Hydrocortisone
Interleukin-6
Interleukins
Oxidoreductases
Receptors, Glucocorticoid
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012; 34:468–483.2. Svendsen PF, Madsbad S, Nilas L, Paulsen SK, Pedersen SB. Expression of 11beta-hydroxysteroid dehydrogenase 1 and 2 in subcutaneous adipose tissue of lean and obese women with and without polycystic ovary syndrome. Int J Obes (Lond). 2009; 33:1249–1256.
Article3. Du H, Liu L, Wang Y, Nakagawa Y, Lyzlov A, Lutfy K, Friedman TC, Peng X, Liu Y. Specific reduction of G6PT may contribute to downregulation of hepatic 11beta-HSD1 in diabetic mice. J Mol Endocrinol. 2013; 50:167–178.
Article4. Wyrwoll CS, Holmes MC, Seckl JR. 11beta-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011; 32:265–286.
Article5. Biedasek K, Andres J, Mai K, Adams S, Spuler S, Fielitz J, Spranger J. Skeletal muscle 11beta-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1. PLoS One. 2011; 6:e16674.
Article6. Wake DJ, Walker BR. 11 beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol Cell Endocrinol. 2004; 215:45–54.
Article7. Arampatzis S, Kadereit B, Schuster D, Balazs Z, Schweizer RA, Frey FJ, Langer T, Odermatt A. Comparative enzymology of 11beta-hydroxysteroid dehydrogenase type 1 from six species. J Mol Endocrinol. 2005; 35:89–101.
Article8. Singh Y, Kotwal N, Menon AS. Endocrine hypertension - Cushing's syndrome. Indian J Endocrinol Metab. 2011; 15:Suppl 4. S313–S316.
Article9. Neary N, Nieman L. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2010; 17:217–223.
Article10. Yeager MP, Pioli PA, Guyre PM. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response. 2011; 9:332–347.
Article11. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335:2–13.
Article12. Chang JH, Lee KJ, Kim SK, Yoo DH, Kang TY. Validity of SW982 synovial cell line for studying the drugs against rheumatoid arthritis in fluvastatin-induced apoptosis signaling model. Indian J Med Res. 2014; 139:117–124.13. Song JS, Kim CH, Heo JY, Cho YS. Rosiglitazone reduces a wide range of proinflammatory profiles in synovial fibroblast SW982 under spheroid culture. Immunol Lett. 2010; 131:81–88.
Article14. Cho YS, Kim CH, Cheon HG. Cell-based assay for screening 11beta-hydroxysteroid dehydrogenase 1 inhibitors. Anal Biochem. 2009; 392:110–116.
Article15. Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, Weeke J, Jorgensen JO, Moller N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007; 92:3553–3559.
Article16. Monder C, Coufalik A. Influence of cortisol on glycogen synthesis and gluconeogenesis in fetal rat liver in organ culture. J Biol Chem. 1972; 247:3608–3617.
Article17. Shultz TD, Bollman S, Kumar R. Decreased intestinal calcium absorption in vivo and normal brush border membrane vesicle calcium uptake in cortisol-treated chickens: evidence for dissociation of calcium absorption from brush border vesicle uptake. Proc Natl Acad Sci U S A. 1982; 79:3542–3546.
Article18. Schwartz M. Glucocorticoids in allergic diseases. Acta Med Scand Suppl. 1969; 500:43–48.
Article19. Alangari AA. Corticosteroids in the treatment of acute asthma. Ann Thorac Med. 2014; 9:187–192.
Article20. Flammer JR, Rogatsky I. Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol. 2011; 25:1075–1086.
Article21. Annane D. Glucocorticoids in the treatment of severe sepsis and septic shock. Curr Opin Crit Care. 2005; 11:449–453.
Article22. Sedwick C. Wanted: a new model for glucocorticoid receptor transactivation and transrepression. PLoS Biol. 2014; 12:e1001814.
Article23. Palacios R, Sugawara I. Hydrocortisone abrogates proliferation of T cells in autologous mixed lymphocyte reaction by rendering the interleukin-2 Producer T cells unresponsive to interleukin-1 and unable to synthesize the T-cell growth factor. Scand J Immunol. 1982; 15:25–31.
Article24. Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011; 286:10265–10275.
Article25. Yamazaki T, Yokoyama T, Akatsu H, Tukiyama T, Tokiwa T. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. In Vitro Cell Dev Biol Anim. 2003; 39:337–339.
Article26. Esteves CL, Verma M, Rog-Zielinska E, Kelly V, Sai S, Breton A, Donadeu FX, Seckl JR, Chapman KE. Pro-inflammatory cytokine induction of 11beta-hydroxysteroid dehydrogenase type 1 in A549 cells requires phosphorylation of C/EBPbeta at Thr235. PLoS One. 2013; 8:e75874.27. Itoi S, Terao M, Murota H, Katayama I. 11beta-Hydroxysteroid dehydrogenase 1 contributes to the proinflammatory response of keratinocytes. Biochem Biophys Res Commun. 2013; 440:265–270.
Article28. Philip AM, Vijayan MM. Stress-immune-growth interactions: cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver. PLoS One. 2015; 10:e0129299.
Article29. Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000; 55:603–613.
Article30. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–1723.
Article31. Castro R, Zou J, Secombes CJ, Martin SA. Cortisol modulates the induction of inflammatory gene expression in a rainbow trout macrophage cell line. Fish Shellfish Immunol. 2011; 30:215–223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition Attenuates the Adverse Effects of Glucocorticoids on Dermal Papilla Cells
- Changes in 11β-Hydroxysteroid Dehydrogenase and Glucocorticoid Receptor Expression in Kawasaki Disease
- Anti-inflammatory effects of rutin in lipopolysaccharide-stimulated canine macrophage cells
- Effect of sildenafil citrate on interleukin-1beta-induced nitric oxide synthesis and iNOS expression in SW982 cells
- Distribution of Synovial Cells in Rabbit Knee Joint