J Nutr Health.
2017 Feb;50(1):10-24. 10.4163/jnh.2017.50.1.10.
Effects of lymphocyte DNA damage levels in Korean plant food groups and Korean diet regarding to glutathione S-transferase M1 and T1 polymorphisms
- Affiliations
-
- 1Department of Food & Nutrition, Daedeok Valley Campus, Hannam University, Daejeon 34054, Korea. mhkang@hnu.kr
- KMID: 2377977
- DOI: http://doi.org/10.4163/jnh.2017.50.1.10
Abstract
- PURPOSE
GST (glutathione S-transferase) M1 and T1 gene polymorphisms are known to affect antioxidant levels. This study was carried out to evaluate genetic susceptibility by measuring the effect of DNA damage reduction in the Korean diet by vegetable food according to GST gene polymorphisms using the ex vivo method with human lymphocytes.
METHODS
Vegetable foods in the Korean diet based the results of the KNHANES V-2 (2011) were classified into 10 food groups. A total of 84 foods, which constituted more than 1% of the total intake in each food group, were finally designated as a vegetable food in the Korean diet. The Korean diet applied in this study is the standard one-week meals for Koreans (2,000 Kcal/day) suggested by the 2010 Dietary Reference Intakes for Koreans. Ex vivo DNA damage in human lymphocytes was assessed using comet assay.
RESULTS
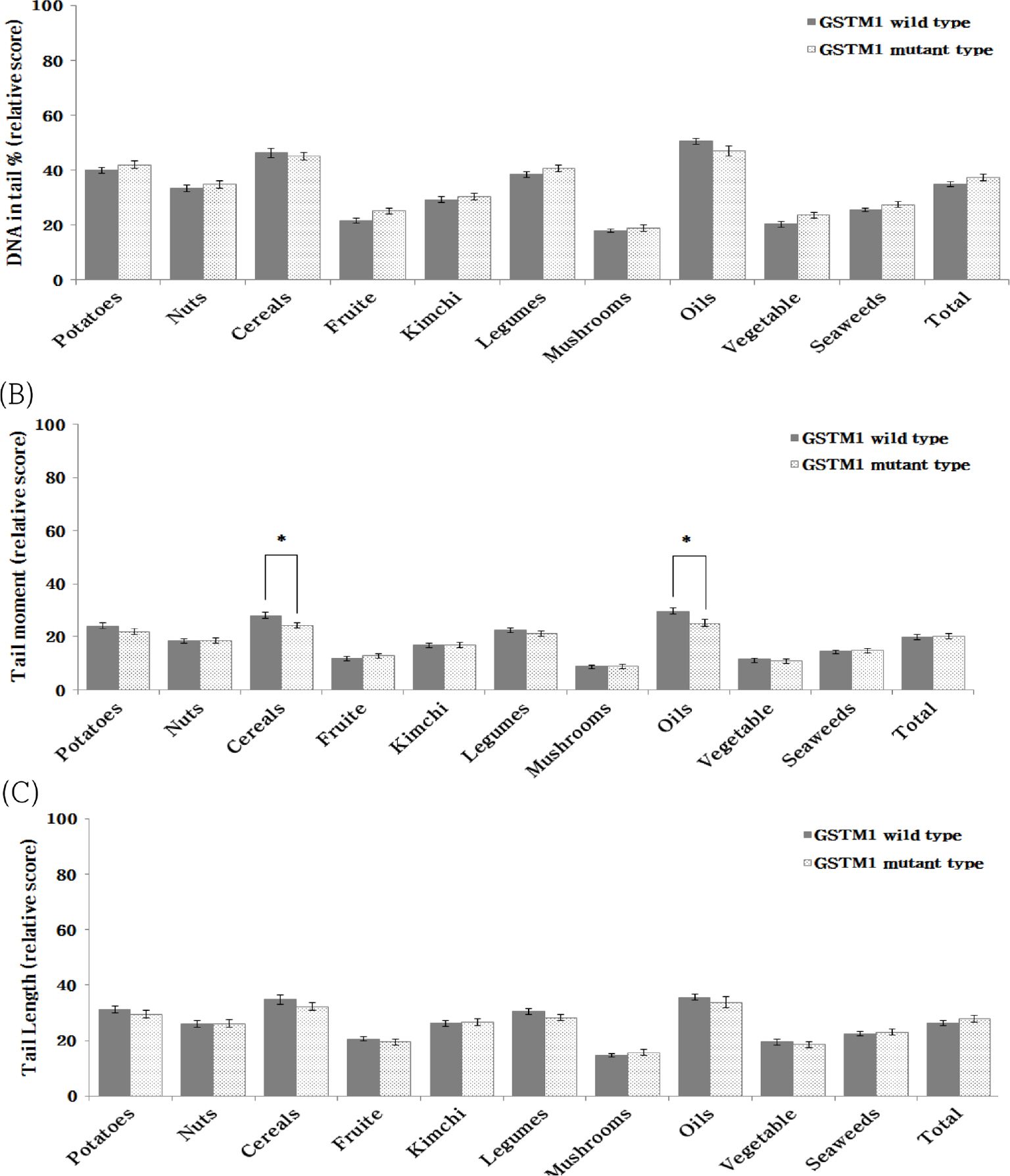
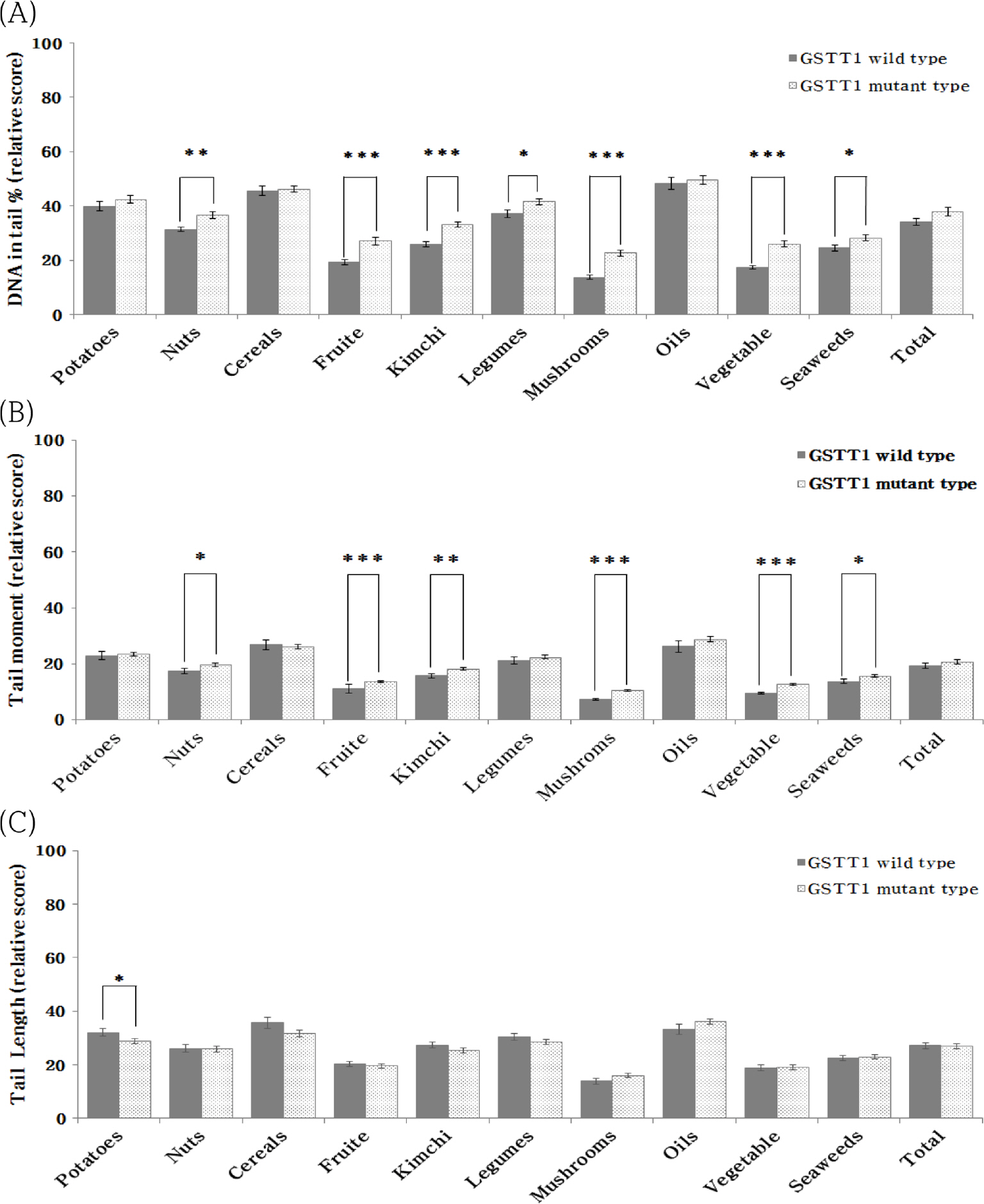
In the Korean food group, the DNA damage protective effect of GSTM1 and GSTT1 was found to be greater in mutant type and wild-type, respectively. and the DNA damage protective effect according to the combined genotype of GSTM1 and GSTT1 was different depending on the food group. On the other hand, in Korean Diet, the DNA damage protective effect appeared to be larger in GSTM1 wild-type than in mutant type and was found to not be affected by GSTT1 genotype.
CONCLUSION
These results can be used as basic data to demonstrate the superiority of the antioxidant function of Korean dietary patterns and food groups. Furthermore, it may be a starting point to begin research on customized antioxidant nutrition according to individual genes.
MeSH Terms
Figure
Reference
-
References
1. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2015: Korea National Health and Nutrition Examination Survey (KNHANES VI-3). Sejong: Korea Centers for Disease Control and Prevention;2016.2. Lee HS, Cho YH, Park J, Shin HR, Sung MK. Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. J Acad Nutr Diet. 2013; 113(9):1194–1199.
Article3. Lee JY. A study on planning the policy for the globalization of Korean food [dissertation]. Seoul: Chung-Ang University;2009.4. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996; 88(21):1550–1559.
Article5. Park CH, Kim KH, Yook HS. Comparison of antioxidant and antimicrobial activities of bracken (Pteridium aquilinum Kuhn) according to cooking methods. Korean J Food Nutr. 2014; 27(3):348–357.
Article6. Lee MY, Han JH, Kang MH. Protective effect of Korean diet food groups on lymphocyte DNA damage and contribution of each food group to total dietary antioxidant capacity (TDAC). J Nutr Health. 2016; 49(5):277–287.
Article7. Lee MY, Kim HA, Kang MH. Comparison of lymphocyte DNA damage levels and total antioxidant capacity in Korean and American diet. Nutr Res Pract. 2017; 11(1):33.
Article8. Park YK, Park E, Kim JS, Kang MH. Daily grape juice consumption reduces oxidative DNA damage and plasma free radical levels in healthy Koreans. Mutat Res. 2003; 529(1–2):77–86.
Article9. Pool-Zobel BL, Bub A, Müller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997; 18(9):1847–1850.
Article10. Sinha R, Caporaso N. Diet, genetic susceptibility and human cancer etiology. J Nutr. 1999; 129(2S Suppl):556S–559S.
Article11. Cho MR, Han JH, Lee HJ, Park YK, Kang MH. Purple grape juice supplementation in smokers and antioxidant status according to different types of GST polymorphisms. J Clin Biochem Nutr. 2015; 56(1):49–56.
Article12. Cho HJ, Lee SY, Ki CS, Kim JW. GSTM1, GSTT1 and GSTP1 polymorphisms in the Korean population. J Korean Med Sci. 2005; 20(6):1089–1092.
Article13. Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1997; 6(9):733–743.14. Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006; 94(3):442–447.
Article15. Han JH, Lee HJ, Cho MR, Chang N, Kim Y, Oh SY, Kang MH. Total antioxidant capacity of the Korean diet. Nutr Res Pract. 2014; 8(2):183–191.
Article16. Hofmann T, Kuhnert A, Schubert A, Gill C, Rowland IR, Pool-Zobel BL, Glei M. Modulation of detoxification enzymes by watercress: in vitro and in vivo investigations in human peripheral blood cells. Eur J Nutr. 2009; 48(8):483–491.
Article17. Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev. 2000; 9(8):787–793.18. Kim SJ, Kim MG, Kim KS, Song JS, Yim SV, Chung JH. Impact of glutathione S-transferase M1 and T1 gene polymorphisms on the smoking-related coronary artery disease. J Korean Med Sci. 2008; 23(3):365–372.
Article19. Yu Y, Song Y. Three clustering patterns among metabolic syndrome risk factors and their associations with dietary factors in Korean adolescents: based on the Korea National Health and Nutrition Examination Survey of 2007–2010. Nutr Res Pract. 2015; 9(2):199–206.
Article20. Woo HD, Shin A, Kim J. Dietary patterns of Korean adults and the prevalence of metabolic syndrome: a cross-sectional study. PLoS One. 2014; 9(11):): e111593.
Article21. The Korean Nutrition Society. Dietary referece intakes for Koreans. 1st rev. ed. Seoul: The Korean Nutrition Society;2010.22. Lee SG, Oh SC, Jang JS. Antioxidant activities of citrus unshiu extracts obtained from different solvents. Korean J Food Nutr. 2015; 28(3):458–464.
Article23. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994; 300(Pt 1):271–276.
Article24. Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993; 85(14):1159–1164.
Article25. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175(1):184–191.
Article26. Riso P, Martini D, M⊘ller P, Loft S, Bonacina G, Moro M, Porrini M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis. 2010; 25(6):595–602.
Article27. Marotta F, Weksler M, Naito Y, Yoshida C, Yoshioka M, Maran-dola P. Nutraceutical supplementation: effect of a fermented papaya preparation on redox status and DNA damage in healthy elderly individuals and relationship with GSTM1 genotype: a randomized, placebocontrolled, crossover study. Ann N Y Acad Sci. 2006; 1067(1):400–407.
Article28. Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005; 82(6):1283–1291.
Article29. Lampe JW. Interindividual differences in response to plant-based diets: implications for cancer risk. Am J Clin Nutr. 2009; 89(5):1553S–1557S.
Article30. Yuan L, Ma W, Liu J, Meng L, Liu J, Li S, Han J, Liu Q, Feng L, Wang C, Xiao R. Effects of GSTM1/GSTT1 gene polymorphism and fruit & vegetable consumption on antioxidant biomarkers and cognitive function in the elderly: a community based cross-sectional study. PLoS One. 2014; 9(11):): e113588.31. Hakim IA, Harris RB, Chow HH, Dean M, Brown S, Ali IU. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: role of glutathione S-transferase genotypes. Cancer Epidemiol Biomarkers Prev. 2004; 13(2):242–249.32. Tang JJ, Wang MW, Jia EZ, Yan JJ, Wang QM, Zhu J, Yang ZJ, Lu X, Wang LS. The common variant in the GSTM1 and GSTT1 genes is related to markers of oxidative stress and inflammation in patients with coronary artery disease: a case-only study. Mol Biol Rep. 2010; 37(1):405–410.
Article33. Wilms LC, Boots AW, de Boer VC, Maas LM, Pachen DM, Gottschalk RW, Ketelslegers HB, Godschalk RW, Haenen GR, van Schooten FJ, Kleinjans JC. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis. 2007; 28(8):1800–1806.
Article34. Wilms LC, Claughton TA, de Kok TM, Kleinjans JC. GSTM1 and GSTT1 polymorphism influences protection against induced oxidative DNA damage by quercetin and ascorbic acid in human lymphocytes in vitro. Food Chem Toxicol. 2007; 45(12):2592–2596.
Article35. Valerio LG Jr, Kepa JK, Pickwell GV, Quattrochi LC. Induction of human NAD(P)H:quinone oxidoreductase (NQO1) gene expression by the flavonol quercetin. Toxicol Lett. 2001; 119(1):49–57.
Article36. Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998; 7(9):775–781.37. Visanji JM, Duthie SJ, Pirie L, Thompson DG, Padfield PJ. Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J Nutr. 2004; 134(11):3121–3126.
Article38. Pool-Zobel B, Veeriah S, Böhmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens – focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005; 591(1–2):74–92.39. Yuan L, Zhang L, Ma W, Zhou X, Ji J, Li N, Xiao R. Glutathione S-transferase M1 and T1 gene polymorphisms with consumption of high fruit-juice and vegetable diet affect antioxidant capacity in healthy adults. Nutrition. 2013; 29(7–8):965–971.
Article40. Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001; 10(10):1063–1067.41. Schroeder N, Park YH, Kang MS, Kim Y, Ha GK, Kim HR, Yates AA, Caballero B. A randomized trial on the effects of 2010 Dietary Guidelines for Americans and Korean diet patterns on cardiovascular risk factors in overweight and obese adults. J Acad Nutr Diet. 2015; 115(7):1083–1092.
Article42. Zamora-Ros R, Serafini M, Estruch R, Lamuela-Raventós RM, Martínez-González MA, Salas-Salvadó J, Fiol M, Lapetra J, Arós F, Covas MI, Andres-Lacueva C. PREDIMED Study Investigators. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: evidence for a mechanism of antioxidant tuning. Nutr Metab Cardiovasc Dis. 2013; 23(12):1167–1174.
Article43. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013; 368(14):1279–1290.
Article44. Chung J, Kwon SO, Ahn H, Hwang H, Hong SJ, Oh SY. Association between dietary patterns and atopic dermatitis in relation to GSTM1 and GSTT1 polymorphisms in young children. Nutrients. 2015; 7(11):9440–9452.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lymphocyte DNA Damage and Anti-Oxidative Parameters are Affected by the Glutathione S-Transferase (GST) M1 and T1 Polymorphism and Smoking Status in Korean Young Adults
- Association between Antipsychotic-Induced Restless Legs Syndrome and Glutathione S-Transferase Gst-M1, Gst-T1 and Gst-P1 Gene Polymorphisms
- Effects of Smoking and Polymorphisms of Glutathione S-Transferase M1 and T1 as Risk Factors on the Development of Bladder Cancer
- Effects of glutathione s-transferase (GST) M1 and T1 polymorphisms on antioxidant vitamins and oxidative stress-related parameters in Korean subclinical hypertensive subjects after kale juice (Brassica oleracea acephala) supplementation
- Benzo[a]pyrene-Induced DNA-Protein Crosslinks in Cultured Human Lymphocytes and the Role of the GSTM1 and GSTT1 Genotypes