World J Mens Health.
2017 Apr;35(1):34-42. 10.5534/wjmh.2017.35.1.34.
The Effect of Alcohol Administration on the Corpus Cavernosum
- Affiliations
-
- 1Department of Urology, Gyeongsang National University Hospital, Jinju, Korea. hyunjs@gnu.ac.kr
- 2Department of Urology, Gyeongsang National University Changwon Hospital, Changwon, Korea.
- KMID: 2377210
- DOI: http://doi.org/10.5534/wjmh.2017.35.1.34
Abstract
- PURPOSE
We studied the effects of alcohol administration on the corpus cavernosum (CC) using an animal model.
MATERIALS AND METHODS
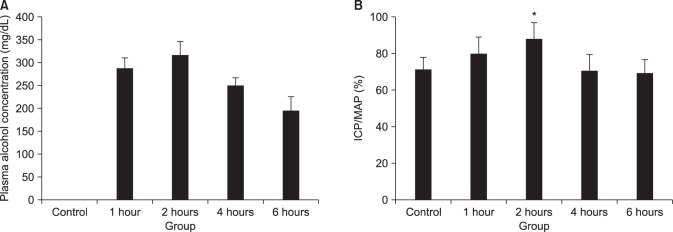
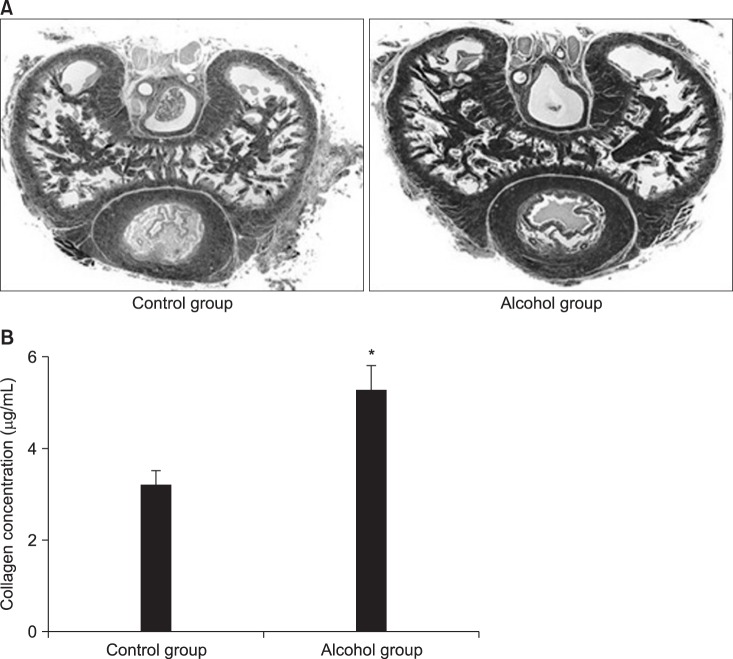
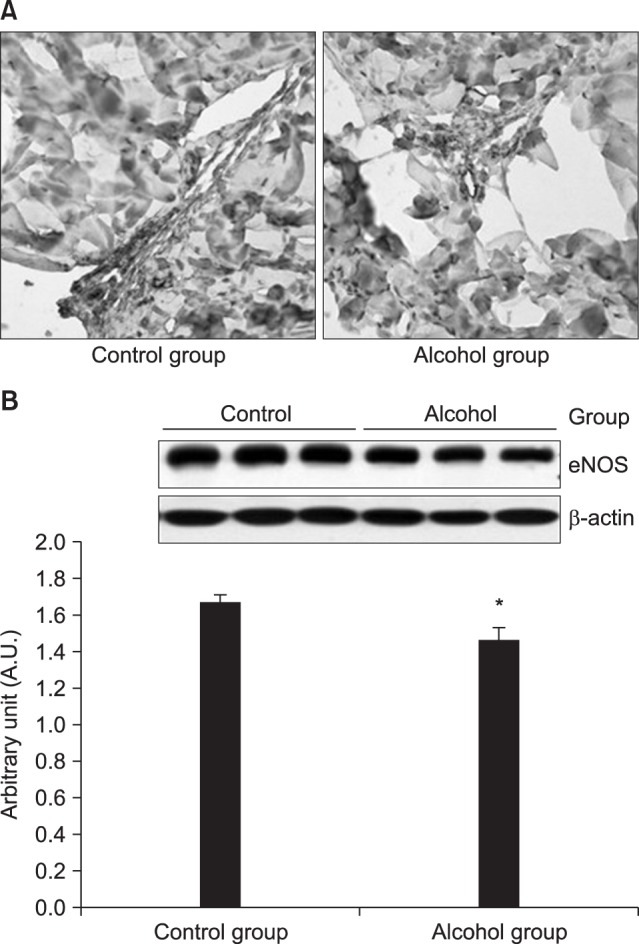
CC sections and the aortic ring of rabbits were used in an organ bath study. After acute alcohol administration, changes in blood alcohol concentration and electrical stimulation induced intracavernosal pressure/mean arterial pressure (ICP/MAP) percentage were compared in rats. Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) levels in the CC were measured using immunoassays. After chronic alcohol administration, ICP/MAP percentage, cAMP and cGMP were compared in rats. Histological changes were examined using the Masson trichrome stain and the Sircol collagen assay. Endothelial nitric oxide synthase (eNOS) expression was examined using immunohistochemistry and Western blotting.
RESULTS
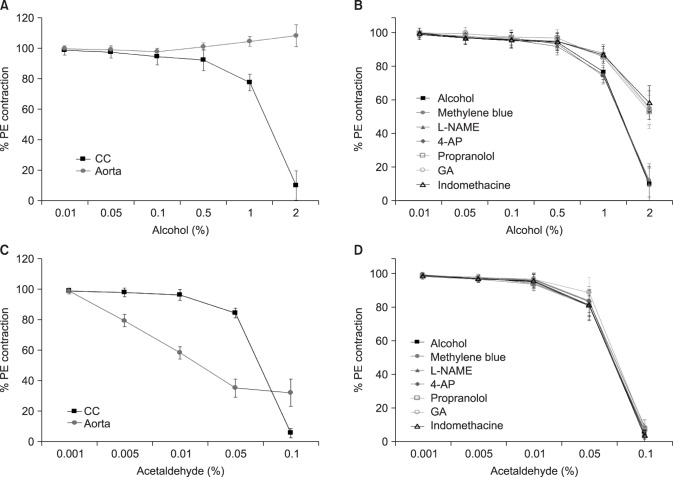
Alcohol relaxed the CC in a dose-dependent manner, and the relaxation response was suppressed when pretreated with propranolol, indomethacin, glibenclamide, and 4-aminopyridine. In rats with acute alcohol exposure, the cAMP level in the CC was significantly greater than was observed in the control group (p<0.05). In rats with chronic alcohol exposure, however, changes in cAMP and cGMP levels were insignificant, and the CC showed markedly smaller areas of smooth muscle, greater amounts of dense collagen (p<0.05). Immunohistochemical analysis of eNOS showed a less intense response, and western blotting showed that eNOS expression was significantly lower in this group (p<0.05).
CONCLUSIONS
Acute alcohol administration activated the cAMP pathway with positive effects on erectile function. In contrast, chronic alcohol administration changed the ultrastructures of the CC and suppressed eNOS expression, thereby leading to erectile dysfunction.
Keyword
MeSH Terms
-
4-Aminopyridine
Adenosine Monophosphate
Animals
Arterial Pressure
Baths
Blood Alcohol Content
Blotting, Western
Collagen
Cyclic AMP
Electric Stimulation
Erectile Dysfunction
Glyburide
Guanosine Monophosphate
Immunoassay
Immunohistochemistry
Indomethacin
Male
Models, Animal
Muscle, Smooth
Nitric Oxide Synthase Type III
Penile Erection
Propranolol
Rabbits
Rats
Relaxation
4-Aminopyridine
Adenosine Monophosphate
Blood Alcohol Content
Collagen
Cyclic AMP
Glyburide
Guanosine Monophosphate
Indomethacin
Nitric Oxide Synthase Type III
Propranolol
Figure
Reference
-
1. Lizza EF, Rosen RC. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res. 1999; 11:141–143. PMID: 10404282.
Article2. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61. PMID: 8254833.
Article3. Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001; 166:569–574. discussion 574-5. PMID: 11458070.
Article4. Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009; 6(Suppl 3):353–362. PMID: 19267860.5. Badr FM, Bartke A, Dalterio S, Bulger W. Suppression of testosterone production by ethyl alcohol. Possible mode of action. Steroids. 1977; 30:647–655. PMID: 611632.
Article6. Leonard MP, Nickel CJ, Morales A. Hyperprolactinemia and impotence: why, when and how to investigate. J Urol. 1989; 142:992–994. PMID: 2795758.
Article7. Borchers H, Brehmer B, Kirschner-Hermanns R, Reineke T, Tietze L, Jakse G. Erectile function after non-nerve-sparing radical prostatectomy: fact or fiction? Urol Int. 2006; 76:213–216. PMID: 16601381.
Article8. Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012; 52:93–104. PMID: 22041278.
Article9. Green GR. Mechanism of hypogonadism in cirrhotic males. Gut. 1977; 18:843–853. PMID: 590844.
Article10. Diederichs W, Stief CG, Lue TF, Tanagho EA. Norepinephrine involvement in penile detumescence. J Urol. 1990; 143:1264–1266. PMID: 2342199.
Article11. Costa P, Soulie-Vassal ML, Sarrazin B, Rebillard X, Navratil H, Bali JP. Adrenergic receptors on smooth muscle cells isolated from human penile corpus cavernosum. J Urol. 1993; 150:859–863. PMID: 8393943.
Article12. Hedlund H, Andersson KE. Comparison of the responses to drugs acting on adrenoreceptors and muscarinic receptors in human isolated corpus cavernosum and cavernous artery. J Auton Pharmacol. 1985; 5:81–88. PMID: 3157689.
Article13. Moreland RB, Kim N, Nehra A, Goldstein I, Traish A. Functional prostaglandin E (EP) receptors in human penile corpus cavernosum. Int J Impot Res. 2003; 15:362–368. PMID: 14562138.
Article14. Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res. 2002; 14(Suppl 1):S17–S21. PMID: 11850730.
Article15. Christ GJ. K+ channels and gap junctions in the modulation of corporal smooth muscle tone. Drug News Perspect. 2000; 13:28–36. PMID: 12937650.16. Archer SL. Potassium channels and erectile dysfunction. Vascul Pharmacol. 2002; 38:61–71. PMID: 12378824.
Article17. Lee SW. Physiological roles and properties of potassium channels in corporal smooth muscle. Drugs Today (Barc). 2000; 36:147–154. PMID: 12879112.
Article18. Christ GJ. K channels as molecular targets for the treatment of erectile dysfunction. J Androl. 2002; 23:S10–S19. PMID: 12236168.19. Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, Pickard R, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005; 2:26–39. PMID: 16422902.20. Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005; 32:379–395. PMID: 16291031.
Article21. Creed KE, Carati CJ, Keogh EJ. The physiology of penile erection. Oxf Rev Reprod Biol. 1991; 13:73–95. PMID: 1688266.23. Lizarte FS, Claudino MA, Tirapelli CR, Morgueti M, Tirapelli DP, Batalhão ME, et al. Chronic ethanol consumption induces cavernosal smooth muscle dysfunction in rats. Urology. 2009; 74:1250–1256. PMID: 19615717.
Article24. Costa WS, Carrerete FB, Horta WG, Sampaio FJ. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006; 97:567–569. PMID: 16469027.
Article25. Shen ZJ, Jin XD, Chen ZD, Shi YH. Effect of aging on penile ultrastructure. Asian J Androl. 2001; 3:281–284. PMID: 11753473.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacologic Effect of Phentolamine on Norepinephrine Induced Contraction of Corpus Cavernosum
- A Case or Priapism Treated by Corpus Cavernosum-Saphenous Vein Anastomosis
- A Fibrotic Nodule in the Corpus Cavernosum
- The Effect and Mechanism of the Specific Phosphodiesterase (PDE) III-Inhibitor Milrinone on Human and Rabbit Corpus Cavernosum
- Effects of Ginseng Alkaloid on the Tension of Rabbit Corpus Cavernosum