Diabetes Metab J.
2017 Apr;41(2):89-95. 10.4093/dmj.2017.41.2.89.
Role of NO/VASP Signaling Pathway against Obesity-Related Inflammation and Insulin Resistance
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. lwjatlas@amc.seoul.kr
- 2Department of Medicine, Diabetes and Obesity Center of Excellence, University of Washington, Seattle, WA, USA.
- KMID: 2376770
- DOI: http://doi.org/10.4093/dmj.2017.41.2.89
Abstract
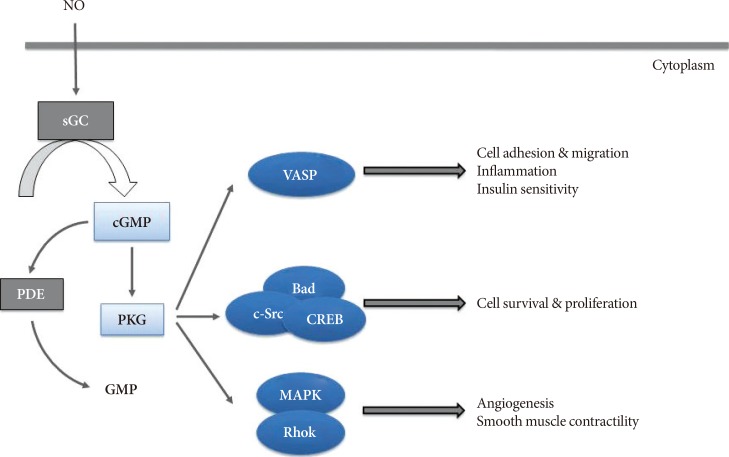
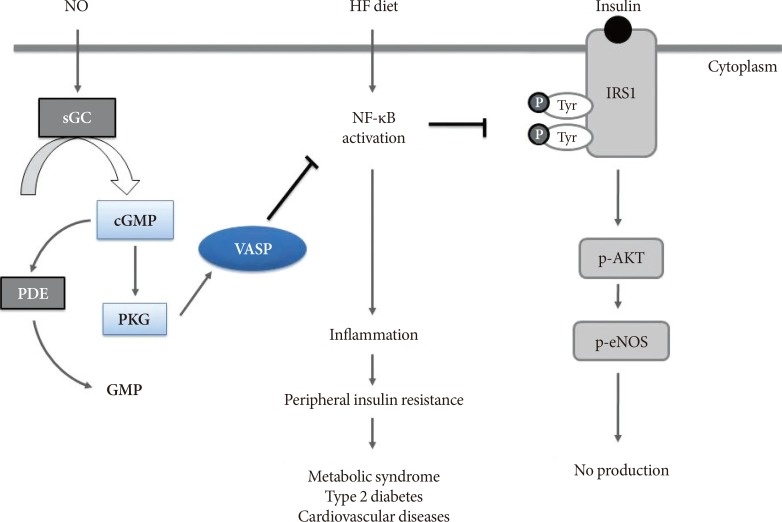
- Obesity has quickly become a worldwide pandemic, causing major adverse health outcomes such as dyslipidemia, type 2 diabetes mellitus, cardiovascular disease and cancers. Obesity-induced insulin resistance is the key for developing these metabolic disorders, and investigation to understand the molecular mechanisms involved has been vibrant for the past few decades. Of these, low-grade chronic inflammation is suggested as a critical concept in the development of obesity-induced insulin resistance, and the anti-inflammatory effect of nitric oxide (NO) signaling has been reported to be linked to improvement of insulin resistance in multiple organs involved in glucose metabolism. Recently, a body of evidence suggested that vasodilatory-stimulated phosphoprotein (VASP), a downstream mediator of NO signaling plays a crucial role in the anti-inflammatory effect and improvement of peripheral insulin resistance. These preclinical studies suggest that NO/VASP signaling could be an ideal therapeutic target in the treatment of obesity-related metabolic dysfunction. In this review, we introduce studies that investigated the protective role of NO/VASP signaling against obesity-related inflammation and insulin resistance in various tissues.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment
Sunghwan Suh, Kwang-Won Kim
Diabetes Metab J. 2019;43(6):733-743. doi: 10.4093/dmj.2019.0177.
Reference
-
1. Korean Diabetes Association. Korean Diabetes Fact Sheet 2015. Seoul: Korean Diabetes Association;2015. p. 1–28.2. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91. PMID: 7678183.3. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009; 6:399–409. PMID: 19399028.
Article4. McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013; 4:52. PMID: 23675368.
Article5. Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med. 2014; 73:383–399. PMID: 24878261.
Article6. Bender SB, Herrick EK, Lott ND, Klabunde RE. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab. 2007; 9:688–696. PMID: 17697061.
Article7. Gruber HJ, Mayer C, Mangge H, Fauler G, Grandits N, Wilders-Truschnig M. Obesity reduces the bioavailability of nitric oxide in juveniles. Int J Obes (Lond). 2008; 32:826–831. PMID: 18197180.
Article8. Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens. 2001; 14:1038–1045. PMID: 11710783.
Article9. Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010; 62:525–563. PMID: 20716671.
Article10. Handa P, Tateya S, Rizzo NO, Cheng AM, Morgan-Stevenson V, Han CY, Clowes AW, Daum G, O'Brien KD, Schwartz MW, Chait A, Kim F. Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding. Arterioscler Thromb Vasc Biol. 2011; 31:2827–2835. PMID: 21903940.
Article11. Tateya S, Rizzo NO, Handa P, Cheng AM, Morgan-Stevenson V, Daum G, Clowes AW, Morton GJ, Schwartz MW, Kim F. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes. 2011; 60:2792–2801. PMID: 21911751.
Article12. Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011; 13:149–159. PMID: 21284982.
Article13. Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011; 89:525–532. PMID: 20937740.
Article14. Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002; 106:2530–2532. PMID: 12427646.
Article15. Price CJ, Brindle NP. Vasodilator-stimulated phosphoprotein is involved in stress-fiber and membrane ruffle formation in endothelial cells. Arterioscler Thromb Vasc Biol. 2000; 20:2051–2056. PMID: 10978248.
Article16. Cheng AM, Rizzo-DeLeon N, Wilson CL, Lee WJ, Tateya S, Clowes AW, Schwartz MW, Kim F. Vasodilator-stimulated phosphoprotein protects against vascular inflammation and insulin resistance. Am J Physiol Endocrinol Metab. 2014; 307:E571–E579. PMID: 25117404.
Article17. Lee WJ, Tateya S, Cheng AM, Rizzo-DeLeon N, Wang NF, Handa P, Wilson CL, Clowes AW, Sweet IR, Bomsztyk K, Schwartz MW, Kim F. M2 macrophage polarization mediates anti-inflammatory effects of endothelial nitric ixide signaling. Diabetes. 2015; 64:2836–2846. PMID: 25845662.18. Tateya S, Rizzo-De Leon N, Handa P, Cheng AM, Morgan-Stevenson V, Ogimoto K, Kanter JE, Bornfeldt KE, Daum G, Clowes AW, Chait A, Kim F. VASP increases hepatic fatty acid oxidation by activating AMPK in mice. Diabetes. 2013; 62:1913–1922. PMID: 23349495.
Article19. Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009; 20:295–302. PMID: 19647446.
Article20. Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008; 77:19–29. PMID: 17658499.21. Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003; 13:386–392. PMID: 12837609.
Article22. Leung EL, Wong JC, Johlfs MG, Tsang BK, Fiscus RR. Protein kinase G type Ialpha activity in human ovarian cancer cells significantly contributes to enhanced Src activation and DNA synthesis/cell proliferation. Mol Cancer Res. 2010; 8:578–591. PMID: 20371672.23. Wong JC, Fiscus RR. Protein kinase G activity prevents pathological-level nitric oxide-induced apoptosis and promotes DNA synthesis/cell proliferation in vascular smooth muscle cells. Cardiovasc Pathol. 2010; 19:e221–e231. PMID: 20060325.
Article24. Wong JC, Fiscus RR. Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Ialpha (PKG-Ialpha) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Ialpha autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells. J Cell Biochem. 2011; 112:829–839. PMID: 21328456.25. Johlfs MG, Fiscus RR. Protein kinase G type-Ialpha phosphorylates the apoptosis-regulating protein Bad at serine 155 and protects against apoptosis in N1E-115 cells. Neurochem Int. 2010; 56:546–553. PMID: 20043968.26. Wong JC, Bathina M, Fiscus RR. Cyclic GMP/protein kinase G type-Ialpha (PKG-Ialpha) signaling pathway promotes CREB phosphorylation and maintains higher c-IAP1, livin, survivin, and Mcl-1 expression and the inhibition of PKG-Ialpha kinase activity synergizes with cisplatin in non-small cell lung cancer cells. J Cell Biochem. 2012; 113:3587–3598. PMID: 22740515.27. Kawasaki K, Smith RS Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003; 23:5726–5737. PMID: 12897144.
Article28. Kirk JA, Holewinski RJ, Crowgey EL, Van Eyk JE. Protein kinase G signaling in cardiac pathophysiology: impact of proteomics on clinical trials. Proteomics. 2016; 16:894–905. PMID: 26670943.
Article29. Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003; 23:26–36. PMID: 12524221.30. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006; 116:1494–1505. PMID: 16691291.
Article31. Westerbacka J, Corner A, Kolak M, Makkonen J, Turpeinen U, Hamsten A, Fisher RM, Yki-Jarvinen H. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am J Physiol Endocrinol Metab. 2008; 294:E841–E845. PMID: 18270300.
Article32. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005; 11:191–198. PMID: 15685170.33. Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. Semin Immunopathol. 2009; 31:399–409. PMID: 19468732.
Article34. Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997; 100:2923–2930. PMID: 9389760.
Article35. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008; 28:1982–1988. PMID: 18772497.
Article36. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749. PMID: 21984069.
Article37. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005; 54:2277–2286. PMID: 16046292.
Article38. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830. PMID: 14679177.
Article39. Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002; 1:1. PMID: 12437787.40. Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010; 298:G107–G116. PMID: 19875703.
Article41. Papackova Z, Palenickova E, Dankova H, Zdychova J, Skop V, Kazdova L, Cahova M. Kupffer cells ameliorate hepatic insulin resistance induced by high-fat diet rich in monounsaturated fatty acids: the evidence for the involvement of alternatively activated macrophages. Nutr Metab (Lond). 2012; 9:22. PMID: 22439764.
Article42. Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006; 22:423–436. PMID: 16506274.
Article43. Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science. 2007; 318:928–929. PMID: 17991852.44. Rizzo NO, Maloney E, Pham M, Luttrell I, Wessells H, Tateya S, Daum G, Handa P, Schwartz MW, Kim F. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler Thromb Vasc Biol. 2010; 30:758–765. PMID: 20093624.
Article45. Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, Isomaa B, Forsen B, Homstrom N, Saloranta C, Taskinen MR, Groop L, Tuomi T. Botnia study group. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005; 54:166–174. PMID: 15616025.
Article