Yonsei Med J.
2016 May;57(3):748-753. 10.3349/ymj.2016.57.3.748.
Efficacy of Anti-NaV1.7 Antibody on the Sensory Nervous System in a Rat Model of Lumbar Intervertebral Disc Injury
- Affiliations
-
- 1Department of Orthopaedic Surgery, Graduate School of Medicine, Chiba University, Chiba, Japan. sohtori@faculty.chiba-u.jp
- KMID: 2374097
- DOI: http://doi.org/10.3349/ymj.2016.57.3.748
Abstract
- PURPOSE
The pathophysiology of discogenic low back pain is not fully understood. Tetrodotoxin-sensitive voltage-gated sodium (NaV) channels are associated with primary sensory nerve transmission, and the NaV1.7 channel has emerged as an analgesic target. Previously, we found increased NaV1.7 expression in dorsal root ganglion (DRG) neurons innervating injured discs. This study aimed to examine the effect of blocking NaV1.7 on sensory nerves after disc injury.
MATERIALS AND METHODS
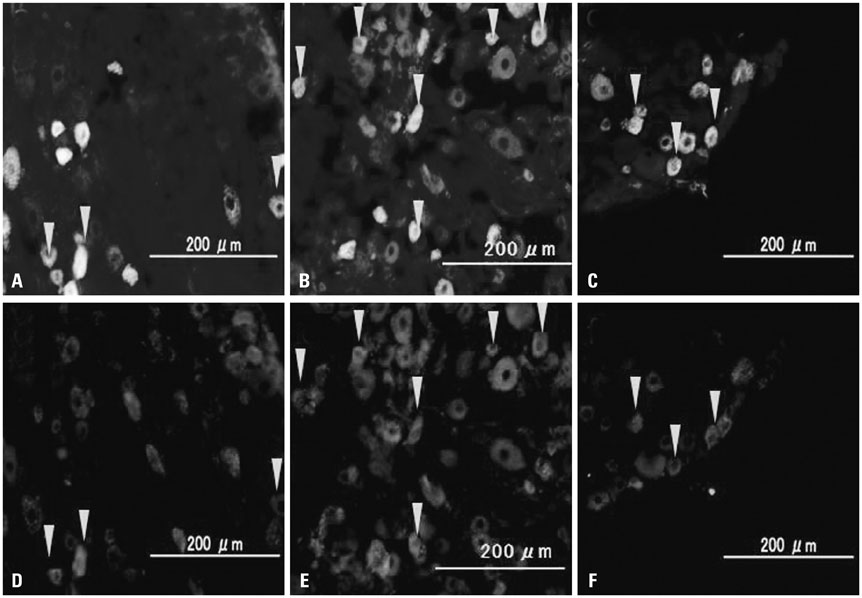
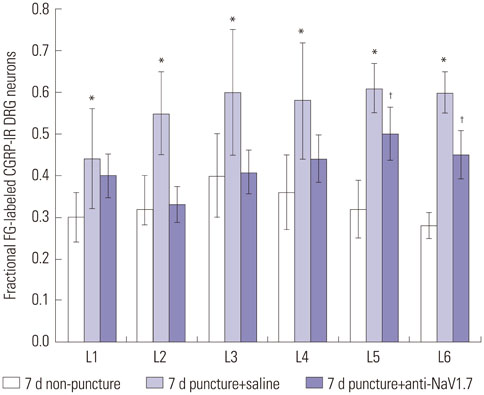
Rat DRG neurons innervating the L5/6 disc were labeled with Fluoro-Gold (FG) neurotracer. Twenty-four rats underwent intervertebral disc puncture (puncture group) and 12 rats underwent sham surgery (non-puncture group). The injury group was divided into a saline infusion group (puncture+saline group) and a NaV1.7 inhibition group, injected with anti-NaV1.7 antibody (puncture+anti-NaV1.7 group); n=12 per group. Seven and 14 days post-surgery, L1 to L6 DRGs were harvested and immunostained for calcitonin gene-related peptide (CGRP) (an inflammatory pain marker), and the proportion of CGRP-immunoreactive (IR) DRG neurons of all FG-positive neurons was evaluated.
RESULTS
The ratio of CGRP-IR DRG neurons to total FG-labeled neurons in the puncture+saline group significantly increased at 7 and 14 days, compared with the non-puncture group, respectively (p<0.05). Application of anti-NaV1.7 into the disc significantly decreased the ratio of CGRP-IR DRG neurons to total FG-labeled neurons after disc puncture at 7 and 14 days (40% and 37%, respectively; p<0.05).
CONCLUSION
NaV1.7 antibody suppressed CGRP expression in disc DRG neurons. Anti-NaV1.7 antibody is a potential therapeutic target for pain control in patients with lumbar disc degeneration.
Keyword
MeSH Terms
-
Animals
Antibodies
Calcitonin Gene-Related Peptide/metabolism
Disease Models, Animal
Ganglia, Spinal/*metabolism
Intervertebral Disc/*drug effects/*injuries
Intervertebral Disc Degeneration/metabolism
Low Back Pain/*physiopathology
Lumbar Vertebrae/injuries
Male
NAV1.7 Voltage-Gated Sodium Channel/*metabolism
Neurons/*metabolism
Pain/metabolism
Rats
Rats, Sprague-Dawley
Stilbamidines
Antibodies
Calcitonin Gene-Related Peptide
Stilbamidines
Figure
Reference
-
1. Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015; 15:1347–1355.
Article2. Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006; 88:Suppl 2. 76–82.
Article3. Ashton IK, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs. Binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994; 65:635–639.
Article4. Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of "painful" lumbar discs. Spine (Phila Pa 1976). 1997; 22:2342–2349.
Article5. Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976). 1995; 20:2645–2651.6. McCarthy PW, Carruthers B, Martin D, Petts P. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine (Phila Pa 1976). 1991; 16:653–655.
Article7. Miyagi M, Ishikawa T, Orita S, Eguchi Y, Kamoda H, Arai G, et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976). 2011; 36:2260–2266.
Article8. Aoki Y, Ohtori S, Ino H, Douya H, Ozawa T, Saito T, et al. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine (Phila Pa 1976). 2004; 29:2621–2626.
Article9. Hayashi Y, Ohtori S, Yamashita M, Yamauchi K, Inoue G, Suzuki M, et al. Direct single injection of p38 mitogen-activated protein kinase inhibitor does not affect calcitonin gene-related peptide expression in dorsal root ganglion neurons innervating punctured discs in rats. Spine (Phila Pa 1976). 2009; 34:2843–2847.
Article10. Rupasinghe DB, Knapp O, Blomster LV, Schmid AB, Adams DJ, King GF, et al. Localization of Nav 1.7 in the normal and injured rodent olfactory system indicates a critical role in olfaction, pheromone sensing and immune function. Channels (Austin). 2012; 6:103–110.
Article11. King GF, Escoubas P, Nicholson GM. Peptide toxins that selectively target insect Na(V) and Ca(V) channels. Channels (Austin). 2008; 2:100–116.12. Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005; 11:555–562.
Article13. Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006; 52:767–774.
Article14. Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006; 444:894–898.
Article15. Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci U S A. 2010; 107:5148–5153.16. Valdes AM, Arden NK, Vaughn FL, Doherty SA, Leaverton PE, Zhang W, et al. Role of the Nav1.7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken). 2011; 63:440–444.
Article17. Sadamasu A, Sakuma Y, Suzuki M, Orita S, Yamauchi K, Inoue G, et al. Upregulation of NaV1.7 in dorsal root ganglia after intervertebral disc injury in rats. Spine (Phila Pa 1976). 2014; 39:E421–E426.
Article18. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2001; 26:946–950.
Article19. Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014; 155:1793–1801.
Article20. Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013; 154:1009–1021.
Article21. Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995; 7:1484–1494.
Article22. Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998; 20:629–632.
Article23. Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003; 460:167–179.
Article24. Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996; 206:33–36.
Article25. Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004; 74:2627–2642.
Article26. Ozawa T, Aoki Y, Ohtori S, Takahashi K, Chiba T, Ino H, et al. The dorsal portion of the lumbar intervertebral disc is innervated primarily by small peptide-containing dorsal root ganglion neurons in rats. Neurosci Lett. 2003; 344:65–67.
Article27. Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004; 355:45–48.
Article28. Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004; 108:237–247.
Article29. Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2008; 12:564–572.
Article30. Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004; 101:12706–12711.
Article31. Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, et al. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther. 2005; 16:271–277.
Article32. Shields SD, Cheng X, Uçeyler N, Sommer C, Dib-Hajj SD, Waxman SG. Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci. 2012; 32:10819–10832.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propriospinal Myoclonus Induced by a Herniated Lumbar Intervertebral Disc at a Young Age: A Case Report
- Radiological Analysis of Aging Changes of the Lumbar Intervertebral Disc
- Radiculopathy Caused by Discal Cyst
- Spontaneous Total Resolution of Severe Lumbar Disc Herniation
- Histological Composition of the Extruded Intervertebral Disc in Lumbar Intervertebral Disc Herniation