Yonsei Med J.
2016 May;57(3):664-673. 10.3349/ymj.2016.57.3.664.
Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy
- Affiliations
-
- 1College of Nursing, Gachon University, Incheon, Korea.
- 2Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 3Division of Pharmacology, Department of Preclinical Science, Faculty of Medicine, Thammasat University, Bangkok, Thailand.
- 4Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 5Department of Anatomy, Korea University College of Medicine, Seoul, Korea.
- KMID: 2374087
- DOI: http://doi.org/10.3349/ymj.2016.57.3.664
Abstract
- PURPOSE
Diabetic nephropathy is a serious complication of type 2 diabetes mellitus, and delaying the development of diabetic nephropathy in patients with diabetes mellitus is very important. In this study, we investigated inflammation, oxidative stress, and lipid metabolism to assess whether curcumin ameliorates diabetic nephropathy.
MATERIALS AND METHODS
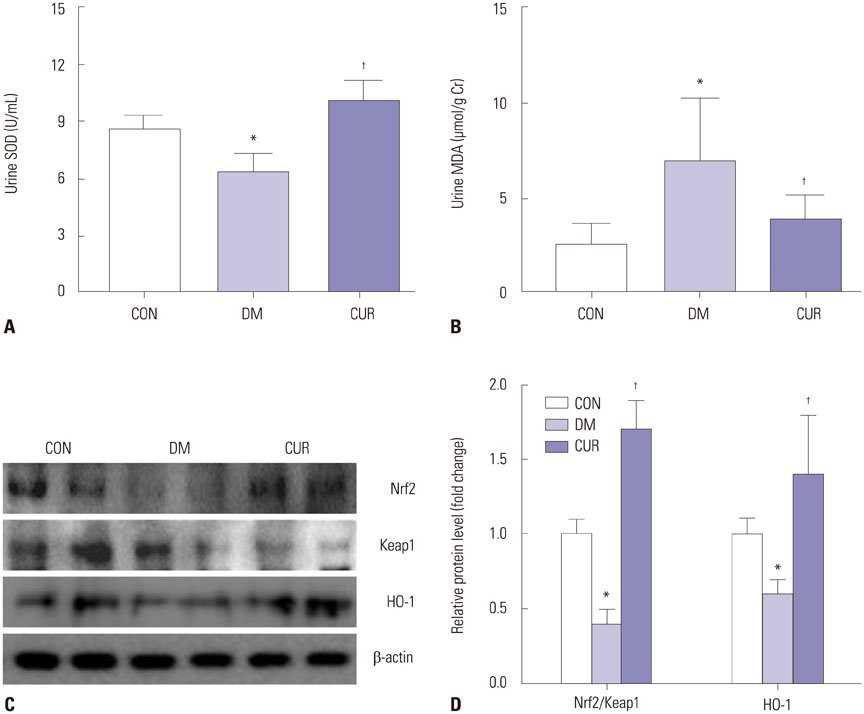
Animals were divided into three groups: Long-Evans-Tokushima-Otsuka rats for normal controls, Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats for the diabetic group, and curcumin-treated (100 mg/kg/day) OLETF rats. We measured body and epididymal fat weights, and examined plasma glucose, adiponectin, and lipid profiles at 45 weeks. To confirm renal damage, we measured albumin-creatinine ratio, superoxide dismutase (SOD), and malondialdehyde (MDA) in urine samples. Glomerular basement membrane thickness and slit pore density were evaluated in the renal cortex tissue of rats. Furthermore, we conducted adenosine monophosphate-activated protein kinase (AMPK) signaling and oxidative stress-related nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling to investigate mechanisms of lipotoxicity in kidneys.
RESULTS
Curcumin ameliorated albuminuria, pathophysiologic changes on the glomerulus, urinary MDA, and urinary SOD related with elevated Nrf2 signaling, as well as serum lipid-related index and ectopic lipid accumulation through activation of AMPK signaling.
CONCLUSION
Collectively, these findings indicate that curcumin exerts renoprotective effects by inhibiting renal lipid accumulation and oxidative stress through AMPK and Nrf2 signaling pathway.
MeSH Terms
-
Albuminuria
Animals
Anti-Inflammatory Agents, Non-Steroidal/*therapeutic use
Curcumin/*pharmacology
Diabetes Mellitus, Type 2/*metabolism/urine
Diabetic Nephropathies/complications/*drug therapy/metabolism/pathology
Gene Expression/drug effects
Inflammation
Kidney/drug effects/metabolism/physiopathology
Kidney Glomerulus/metabolism/physiopathology
Lipid Metabolism/*drug effects
Male
Malondialdehyde/metabolism/urine
Oxidative Stress/*drug effects
Rats
Rats, Inbred OLETF
Rats, Long-Evans
Superoxide Dismutase/metabolism
Anti-Inflammatory Agents, Non-Steroidal
Curcumin
Malondialdehyde
Superoxide Dismutase
Figure
Reference
-
1. Kume S, Koya D, Uzu T, Maegawa H. Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int. 2014; 2014:315494.
Article2. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28:164–176.
Article3. Zelmanovitz T, Gerchman F, Balthazar AP, Thomazelli FC, Matos JD, Canani LH. Diabetic nephropathy. Diabetol Metab Syndr. 2009; 1:10.
Article4. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008; 233:4–11.
Article5. Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005; 54:2328–2335.
Article6. Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, et al. Ectopic lipid accumulation: a potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013; 95:1971–1979.
Article7. de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014; 2:417–426.
Article8. Tesch GH, Lim AK. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2011; 300:F301–F310.9. Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol. 2013; 366:152–162.
Article10. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012; 13:251–262.
Article11. Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007; 292:F617–F627.
Article12. Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, et al. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal. 2010; 22:65–70.
Article13. Lee HJ, Mariappan MM, Feliers D, Cavaglieri RC, Sataranatarajan K, Abboud HE, et al. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem. 2012; 287:4451–4461.
Article14. Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013; 2013:636053.
Article15. Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, et al. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. 2013; 18:756–763.
Article16. DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004; 53:762–768.
Article17. Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011; 31:58–66.
Article18. Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014; 27:586–595.
Article19. Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008; 325:655–664.
Article20. Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006; 536:256–261.
Article21. Huang J, Huang K, Lan T, Xie X, Shen X, Liu P, et al. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol. 2013; 365:231–240.
Article22. Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006; 33:940–945.
Article23. Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011; 27:123–130.
Article24. Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010; 10:57.
Article25. Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007; 577:183–191.
Article26. Sreejayan , Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994; 46:1013–1016.
Article27. Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E. Protective effect of curcumin against contrast induced nephropathy in rat kidney: what is happening to oxidative stress, inflammation, autophagy and apoptosis? Eur Rev Med Pharmacol Sci. 2014; 18:461–470.28. Suckow BK, Suckow MA. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. Int J Biomed Sci. 2006; 2:402–405.29. Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, et al. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr). 2013; 35:1133–1142.
Article30. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003; 371(Pt 3):887–895.
Article31. Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013; 39:101–121.
Article32. Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007; 29:881–889.
Article33. Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Harima M, Suzuki K, et al. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J Nutr Biochem. 2013; 24:796–802.
Article34. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–1428.
Article35. Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition. 2009; 25:964–972.
Article36. Asai A, Nakagawa K, Miyazawa T. Antioxidative effects of turmeric, rosemary and capsicum extracts on membrane phospholipid peroxidation and liver lipid metabolism in mice. Biosci Biotechnol Biochem. 1999; 63:2118–2122.
Article37. Dadras F, Khoshjou F. NF-E2-related factor 2 and its role in diabetic nephropathy. Iran J Kidney Dis. 2013; 7:346–351.38. Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010; 59:850–860.
Article39. Smyth R, Lane CS, Ashiq R, Turton JA, Clarke CJ, Dare TO, et al. Proteomic investigation of urinary markers of carbon-tetrachloride-induced hepatic fibrosis in the Hanover Wistar rat. Cell Biol Toxicol. 2009; 25:499–512.
Article40. Liu D, Huang P, Li X, Ge M, Luo G, Hei Z. Using inflammatory and oxidative biomarkers in urine to predict early acute kidney injury in patients undergoing liver transplantation. Biomarkers. 2014; 19:424–429.
Article41. Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006; 55:2502–2509.
Article42. Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002; 277:18919–18927.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effects of Lithospermate B on Diabetic Nephropathy in OLETF Rat
- Evaluating Pharmacological Effects of Two Major Components of Shuangdan Oral Liquid: Role of Danshensu and Paeonol in Diabetic Nephropathy Rat
- Adenosine monophosphate-activated protein kinase in diabetic nephropathy
- Effects of Spironolactone and Losartan on Diabetic Nephropathy in a Type 2 Diabetic Rat Model
- Blockade of Oxidative Stress by Vitamin C Ameliorates Albuminuria and Renal Sclerosis in Experimental Diabetic Rats