Diabetes Metab J.
2011 Apr;35(2):130-137. 10.4093/dmj.2011.35.2.130.
Effects of Spironolactone and Losartan on Diabetic Nephropathy in a Type 2 Diabetic Rat Model
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 2Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 5Department of Internal Medicine, Sun General Hospital, Daejeon, Korea.
- KMID: 2281711
- DOI: http://doi.org/10.4093/dmj.2011.35.2.130
Abstract
- BACKGROUND
While there is an evidence that the anti-inflammatory properties of spironolactone can attenuate proteinuria in type 2 diabetes, its effects on vascular endothelial growth factor (VEGF) expression in diabetic nephropathy have not been clearly defined. In this study, we examined the effects of spironolactone, losartan, and a combination of these two drugs on albuminuria, renal VEGF expression, and inflammatory and oxidative stress markers in a type 2 diabetic rat model.
METHODS
Thirty-three Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats were divided into four groups and treated with different medication regimens from weeks 25 to 50; OLETF diabetic controls (n=5), spironolactone-treated (n=10), losartan-treated (n=9), and combination of spironolactone- and losartan-treated (n=9).
RESULTS
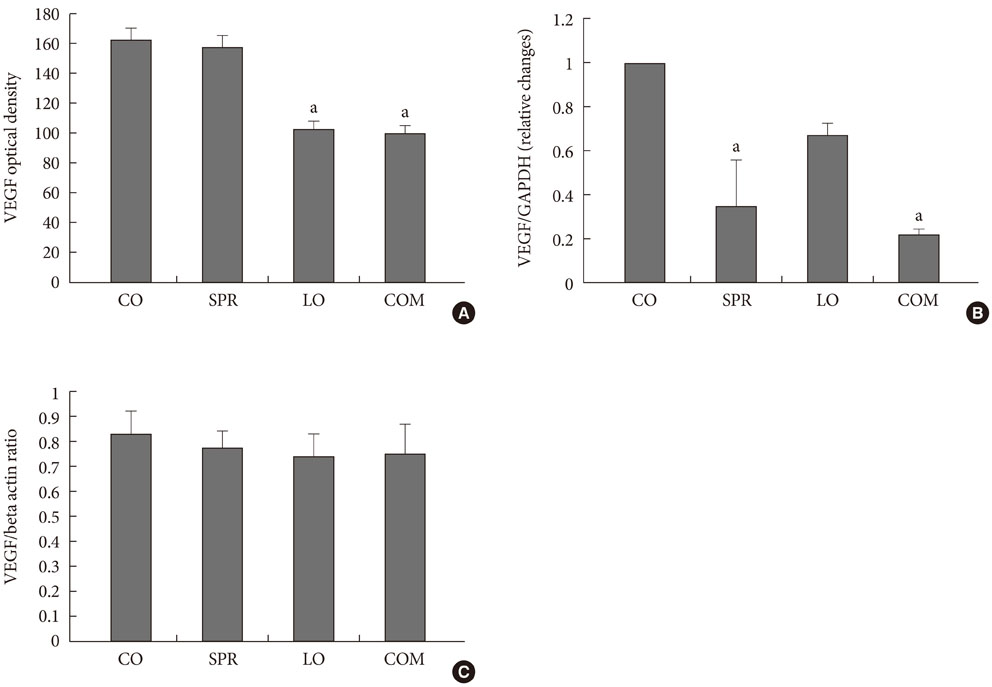
At week 50, the albumin-to-creatinine ratio was significantly decreased in the losartan and combination groups compared to the control OLETF group. No decrease was detected in the spironolactone group. There was a significant reduction in renal VEGF, transforming growth factor (TGF)-beta, and type IV collagen mRNA levels in the spironolactone- and combination regimen-treated groups. Twenty-four hour urine monocyte chemotactic protein-1 levels were comparable in all four groups but did show a decreasing trend in the losartan and combination regimen groups. Twenty-four hour urine malondialdehyde levels were significantly decreased in the spironolactone- and combination regimen-treated groups.
CONCLUSION
These results suggest that losartan alone and a combined regimen of spironolactone and losartan could ameliorate albuninuria by reducing renal VEGF expression. Also, simultaneous treatment with spironolactone and losartan may have protective effects against diabetic nephropathy by decreasing TGF-beta and type IV collagen expression and by reducing oxidative stress in a type 2 diabetic rat model.
MeSH Terms
-
Albuminuria
Animals
Chemokine CCL2
Collagen Type IV
Diabetic Nephropathies
Losartan
Malondialdehyde
Oxidative Stress
Proteinuria
Rats
RNA, Messenger
Spironolactone
Transforming Growth Factor beta
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Chemokine CCL2
Collagen Type IV
Losartan
Malondialdehyde
RNA, Messenger
Spironolactone
Transforming Growth Factor beta
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Brown NJ, Vaughan DE, Fogo AB. Aldosterone and PAI-1: implications for renal injury. J Nephrol. 2002. 15:230–235.2. Stier CT Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev. 2002. 10:97–107.3. Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau JL, Rutherford J, Wertheimer JH, Morton Hawkins C. SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med. 1992. 327:669–677.4. Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994. 89:2273–2282.5. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001. 345:861–869.6. Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001. 345:870–878.7. Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zeni P, Caruso B, Zardini P. Relation of aldosterone "escape" despite angiotensin-converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002. 89:403–407.8. Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003. 41:64–68.9. Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006. 147:5363–5373.10. Han KH, Kang YS, Han SY, Jee YH, Lee MH, Han JY, Kim HK, Kim YS, Cha DR. Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 2006. 70:111–120.11. Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005. 18:44–49.12. Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002. 29:6 Suppl 16. 3–9.13. Moehler TM, Ho AD, Goldschmidt H, Barlogie B. Angiogenesis in hematologic malignancies. Crit Rev Oncol Hematol. 2003. 45:227–244.14. The EUCLID Study Group. Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997. 349:1787–1792.15. Lee EY, Shim MS, Kim MJ, Hong SY, Shin YG, Chung CH. Angiotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med. 2004. 36:65–70.16. Nagisa Y, Shintani A, Nakagawa S. The angiotensin II receptor antagonist candesartan cilexetil (TCV-116) ameliorates retinal disorders in rats. Diabetologia. 2001. 44:883–888.17. Tojo A, Kimura K, Nanba S, Matsuoka H, Sugimoto T. Variations in renal arteriolar diameter in deoxycorticosterone acetate-salt hypertensive rats. A microvascular cast study. Virchows Arch A Pathol Anat Histopathol. 1990. 417:389–393.18. Korchazhkina O, Exley C, Andrew Spencer S. Measurement by reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatisation with 2,4-dinitrophenylhydrazine. J Chromatogr B Analyt Technol Biomed Life Sci. 2003. 794:353–362.19. Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002. 346:1145–1151.20. Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006. 17:1362–1372.21. Nakhoul F, Khankin E, Yaccob A, Kawachi H, Karram T, Awaad H, Nakhoul N, Hoffman A, Abassi Z. Eplerenone potentiates the antiproteinuric effects of enalapril in experimental nephrotic syndrome. Am J Physiol Renal Physiol. 2008. 294:F628–F637.22. Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak M, Ritz E. Regression of glomerulosclerosis in subtotally nephrectomized rats: effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol Renal Physiol. 2008. 295:F137–F144.23. Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, Bonventre JV, Gamba G, Bobadilla NA. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2007. 292:F131–F139.24. Bobadilla NA, Gamba G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. Am J Physiol Renal Physiol. 2007. 293:F2–F9.25. Kelly DJ, Aaltonen P, Cox AJ, Rumble JR, Langham R, Panagiotopoulos S, Jerums G, Holthofer H, Gilbert RE. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant. 2002. 17:1327–1332.26. Williams B, Baker AQ, Gallacher B, Lodwick D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension. 1995. 25:913–917.27. Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003. 162:1123–1137.28. Kakizawa H, Itoh Y, Imamura S, Matsumoto T, Ishiwata Y, Ono Y, Yamamoto K, Kato T, Hayakawa N, Oda N, Goto Y, Goto Y, Nagasaka A, Senda T, Itoh M. Possible role of VEGF in the progression of kidney disease in streptozotocin (STZ)-induced diabetic rats: effects of an ACE inhibitor and an angiotensin II receptor antagonist. Horm Metab Res. 2004. 36:458–464.29. Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab. 2005. 288:E353–E359.30. Kelly DJ, Wilkinson-Berka JL, Ricardo SD, Cox AJ, Gilbert RE. Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2)27 rats. Nephrol Dial Transplant. 2002. 17:985–991.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Spironolactone on Progression of Nephropathy in Type 2 Diabetic Rat Model
- Aldosterone Receptor Blockade Prevents Inflammatory Reaction on Type 2 Diabetic Nephropathy
- Glycemic Control in Diabetic Patients with Diabetic Nephropathy
- Aldose Reductase Inhibitor Ameliorates Renal Vascular Endothelial Growth Factor Expression in Streptozotocin-Induced Diabetic Rats
- Effects of Spironolactone on the Urinary Excretion of TGF-beta1 in IgA Nephropathy