Korean J Gastroenterol.
2015 Oct;66(4):231-236. 10.4166/kjg.2015.66.4.231.
A Case of Crohn's Disease Showing Favorable Response to Induction and Maintenance Therapy with Methotrexate after Failure of Anti-tumor Necrosis Factor Therapy
- Affiliations
-
- 1Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jaejpark@yush.ac
- 2Department of Medicine, Yonsei University Graduate School, Seoul, Korea.
- KMID: 2373347
- DOI: http://doi.org/10.4166/kjg.2015.66.4.231
Abstract
- Thanks to the introduction of immumomodulators and biologics, therapeutic approaches in Crohn's disease have changed significantly during the past decade. Although new biologic therapy has dramatically improved the treatment of Crohn's disease, a substantial number of patients are refractory to these therapies or lose their initial response. Methotrexate (MTX) is a structural analogue of folic acid that can competitively inhibit the binding of dihydrofolic acid to the enzyme dihydrofolate reductase and has been widely used as immunomodulator in rheumatology area for patients with rheumatoid arthritis and psoriasis. Although MTX has also been shown to be an effective agent for remission induction and maintenance of remission in Crohn's disease, the use of MTX in Crohn's disease has not yet been reported in Korea. Herein, we report a case of Crohn's disease patient who was successfully treated with MTX after treatment failure with thiopurine and anti-tumor necrosis factor.
Keyword
MeSH Terms
-
Adult
Antibodies, Monoclonal/therapeutic use
Colonoscopy
Crohn Disease/diagnosis/*drug therapy
Humans
Immunosuppressive Agents/*therapeutic use
Infliximab/therapeutic use
Male
Methotrexate/*therapeutic use
Remission Induction
Tomography, X-Ray Computed
Tumor Necrosis Factor-alpha/immunology
Antibodies, Monoclonal
Immunosuppressive Agents
Infliximab
Methotrexate
Tumor Necrosis Factor-alpha
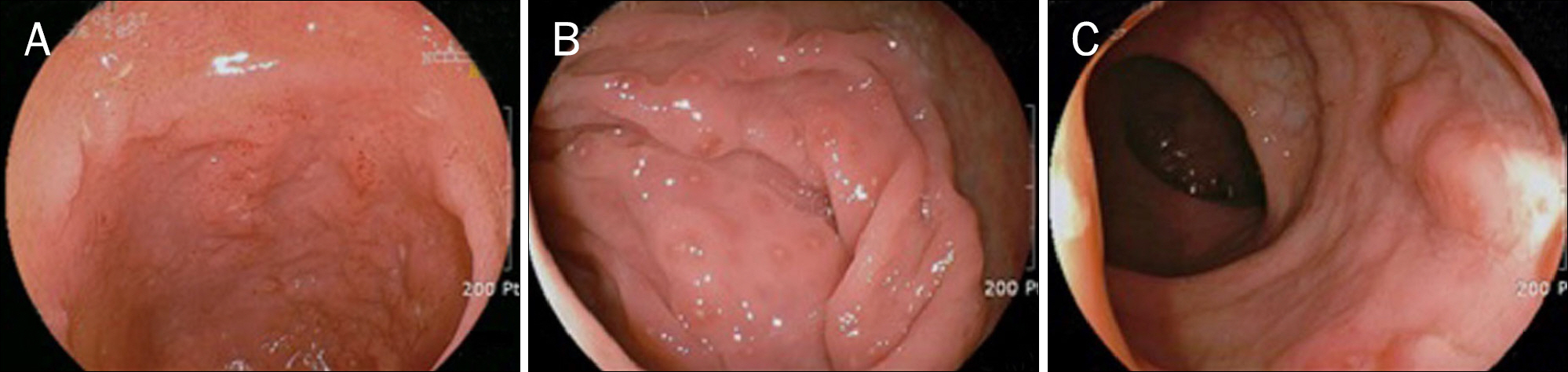
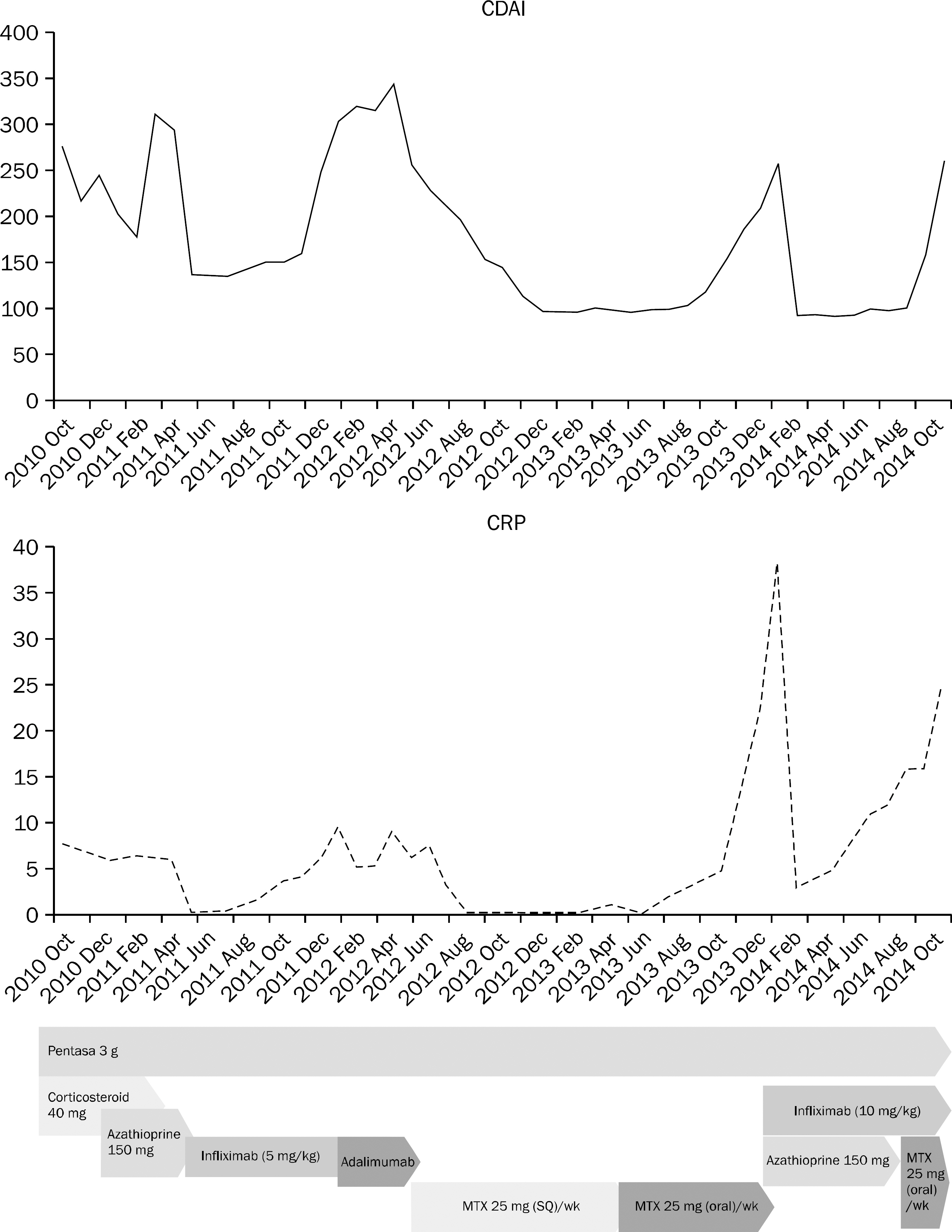
Figure
Reference
-
References
1. Yang SK. Current status and clinical characteristics of inflammatory bowel disease in Korea. Korean J Gastroenterol. 2002; 40:1–14.2. Fraser AG. Methotrexate: first-line or second-line immunomo-dulator? Eur J Gastroenterol Hepatol. 2003; 15:225–231.3. Feagan BG, Rochon J, Fedorak RN, et al. The North American Crohn's Study Group Investigators. Methotrexate for the treatment of Crohn's disease. N Engl J Med. 1995; 332:292–297.
Article4. Chong RY, Hanauer SB, Cohen RD. Efficacy of parenteral methotrexate in refractory Crohn's disease. Aliment Pharmacol Ther. 2001; 15:35–44.
Article5. Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997; 23:739–755.
Article6. Barrera P, Haagsma CJ, Boerbooms AM, et al. Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol. 1995; 34:747–755.
Article7. Ye BD, Yang SK, Shin SJ, et al. IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Guidelines for the management of Crohn's disease. Korean J Gastroenterol. 2012; 59:141–179.
Article8. Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989; 110:353–356.
Article9. Lémann M, Zenjari T, Bouhnik Y, et al. Methotrexate in Crohn's disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000; 95:1730–1734.
Article10. Fraser AG, Morton D, McGovern D, Travis S, Jewell DP. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther. 2002; 16:693–697.
Article11. Jundt JW, Browne BA, Fiocco GP, Steele AD, Mock D. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol. 1993; 20:1845–1849.12. Wilson A, Patel V, Chande N, et al. Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn's disease. Aliment Pharmacol Ther. 2013; 37:340–345.
Article13. Morgan SL, Baggott JE, Vaughn WH, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994; 121:833–841.
Article14. van Ede AE, Laan RF, Rood MJ, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001; 44:1515–1524.15. Said S, Jeffes EW, Weinstein GD. Methotrexate. Clin Dermatol. 1997; 15:781–797.
Article16. Kremer JM, Alarcón GS, Lightfoot RW Jr, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994; 37:316–328.17. Te HS, Schiano TD, Kuan SF, Hanauer SB, Conjeevaram HS, Baker AL. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000; 95:3150–3156.
Article18. Khanna R, Feagan BG. Current and future status of therapeutic drug monitoring in the treatment of IBD. Curr Treat Options Gastroenterol. 2014; 12:76–89.
Article19. Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011; 9:36–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trends of Infliximab Treatment for Crohn's Disease
- Biological Therapy for Inflammatory Bowel Disease in Children
- Preoperative use of anti-tumor necrosis factor therapy in Crohn's disease: promises and pitfalls
- Anti-tumor Necrosis Factor Therapy for Crohn Disease: Friend or Foe to the Surgeon?
- Medical Treatment of Vasculitis; Anti-TNF-alpha Treatment