J Gastric Cancer.
2014 Jun;14(2):123-128.
At Which Stage of Gastric Cancer Progression Do Levels of Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Increase? Application in Advanced Gastric Cancer Treatment
- Affiliations
-
- 1Division of Gastrointestinal Surgery, Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. painkiller9@catholic.ac.kr
Abstract
- PURPOSE
Since there are no proven tumor markers that reflect the course of gastric cancer, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are commonly used alternatives. However, the degree of progression that corresponds to an increase in these markers, and the values of these markers at different cancer stages, remains unclear.
MATERIALS AND METHODS
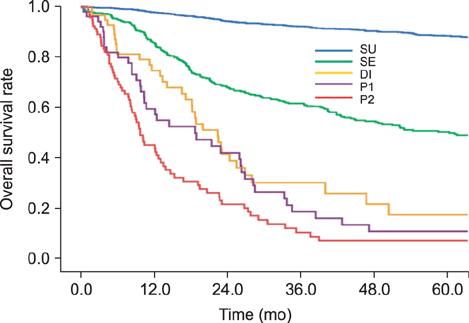
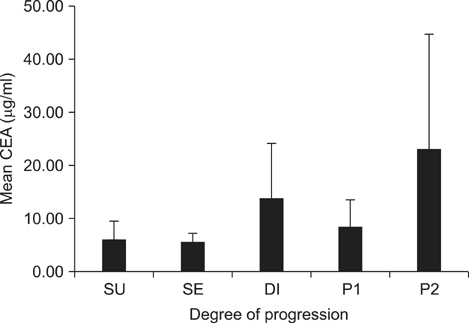
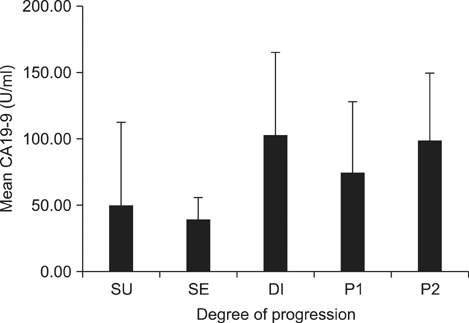
This study enrolled 1,733 gastric cancer patients who underwent surgery and whose pre-operative CEA and CA19-9 levels were known. Survival curves and mean values of the two markers were compared according to the degree of cancer progression: serosa-unexposed (SU), serosa-exposed (SE), direct invasion (DI), localized seeding (P1), and extensive seeding (P2).
RESULTS
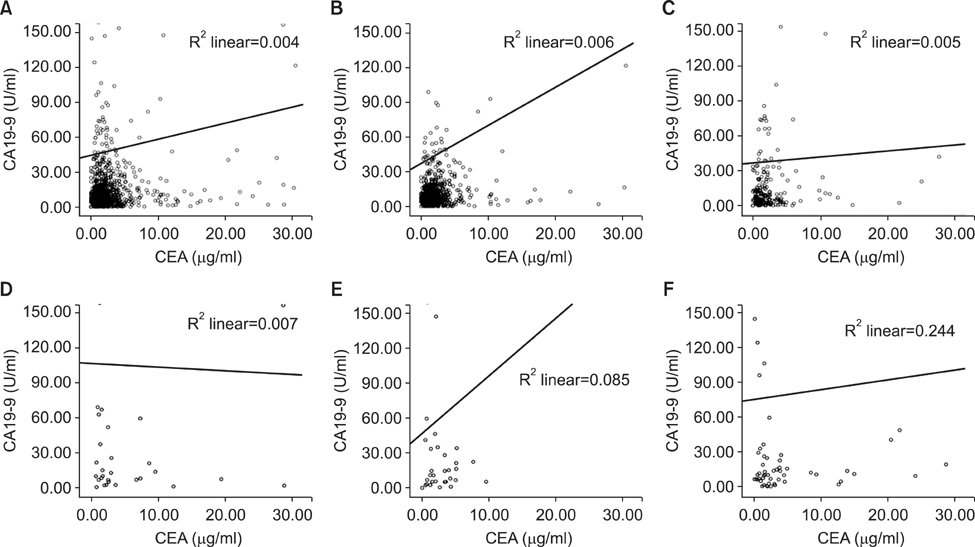
The 5-year overall survival rates at each stage differed significantly, except between DI and P1 patients (17.1% vs. 10.5%, P=0.344). The mean CEA values in SU, SE, DI, P1, and P2 patients were 5.80, 5.48, 13.36, 8.06, and 22.82, respectively. The CA19-9 values for these patients were 49.40, 38.97, 101.67, 73.77, and 98.57, respectively. The increase in CEA in P2 patients was statistically significant (P=0.002), and the increases in CA19-9 in DI and P2 patients were significant (P=0.025, 0.007, respectively). There was a fair correlation between the two markers in P2 patients (r=0.494, P<0.001).
CONCLUSIONS
CA19-9 can be used to assess DI of gastric cancer into adjacent organs. Both markers are useful for predicting the presence of extensive peritoneal seeding.
Keyword
MeSH Terms
Figure
Reference
-
1. Sikaroodi M, Galachiantz Y, Baranova A. Tumor markers: the potential of "omics" approach. Curr Mol Med. 2010; 10:249–257.2. Seregni E, Ferrari L, Martinetti A, Bombardieri E. Diagnostic and prognostic tumor markers in the gastrointestinal tract. Semin Surg Oncol. 2001; 20:147–166.3. Duffy MJ. Role of tumor markers in patients with solid cancers: a critical review. Eur J Intern Med. 2007; 18:175–184.4. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005; 5:845–856.
Article5. Meany DL, Sokoll LJ, Chan DW. Early detection of cancer: immunoassays for plasma tumor markers. Expert Opin Med Diagn. 2009; 3:597–605.
Article6. Fan B, Xiong B. Investigation of serum tumor markers in the diagnosis of gastric cancer. Hepatogastroenterology. 2011; 58:239–245.
Article7. Dilege E, Mihmanli M, Demir U, Ozer K, Bostanci O, Kaya C, et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010; 57:674–677.
Article8. Reiter W, Stieber P, Reuter C, Nagel D, Cramer C, Pahl H, et al. Prognostic value of preoperative serum levels of CEA, CA 19-9 and CA 72-4 in gastric carcinoma. Anticancer Res. 1997; 17:2903–2906.
Article9. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014; 17:26–33.
Article10. Leja M, Wex T, Malfertheiner P. Markers for gastric cancer premalignant lesions: where do we go? Dig Dis. 2012; 30:268–276.
Article11. Basbug M, Arikanoglu Z, Bulbuller N, Cetinkaya Z, Aygen E, Akbulut S, et al. Prognostic value of preoperative CEA and CA 19-9 levels in patients with colorectal cancer. Hepatogastroenterology. 2011; 58:400–405.
Article12. Eskelinen M, Pasanen P, Kulju A, Janatuinen E, Miettinen P, Poikolainen E, et al. Clinical evaluation of serum tumour markers CEA, CA 50 and CA 242 in colorectal cancer. Anticancer Res. 1994; 14:1427–1432.
Article13. Irvine T, Scott M, Clark CI. A small rise in CEA is sensitive for recurrence after surgery for colorectal cancer. Colorectal Dis. 2007; 9:527–531.
Article14. Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, et al. NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012; 23:1713–1722.
Article15. Maisey NR, Norman AR, Hill A, Massey A, Oates J, Cunningham D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer. 2005; 93:740–743.
Article16. Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, et al. Prognostic values of preoperative and postoperative CEA and CA19.9 levels in pancreatic cancer. Pancreas. 1994; 9:735–740.
Article17. Chen S, Feng XY, Li YF, Zhao BW, Zhou ZW, Chen YB. The prognosis of gastric cancer patients with marginally elevated carcinoembryonic antigen (CEA) values after D2 radical gastrectomy. J Surg Oncol. 2013; 107:641–645.
Article18. Park SS, Min JS, Lee KJ, Jin SH, Park S, Bang HY, et al. Risk stratification for serosal invasion using preoperative predictors in patients with advanced gastric cancer. J Gastric Cancer. 2012; 12:149–155.
Article19. Kang Y, Wang F, Zu H, Yang Z, Xue Y. A new subclassification of pT4 gastric cancers according to the width of serosal invasion. PLoS One. 2013; 8:e68042.
Article20. Shim JH, Yoo HM, Lee HH, Kim JG, Jeon HM, Song KY, et al. Use of laparoscopy as an alternative to computed tomography (CT) and positron emission tomography (PET) scans for the detection of recurrence in patients with gastric cancer: a pilot study. Surg Endosc. 2011; 25:3338–3344.
Article21. Hur H, Lee HH, Jung H, Song KY, Jeon HM, Park CH. Predicting factors of unexpected peritoneal seeding in locally advanced gastric cancer: indications for staging laparoscopy. J Surg Oncol. 2010; 102:753–757.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Value of Postoperative Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Levels for the Early Detection of Gastric Cancer Recurrence after Curative Resection
- Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer
- Significance of Carcinoembryonic Antigen Levels in Peritoneal Washings for Gastric Cancer Patients
- Prognostic Factors on Overall Survival in Lymph Node Negative Gastric Cancer Patients Who Underwent Curative Resection
- Prognostic Significance of Serum and Tissue Carcinoembryonic Antigen in Patients with Gastric Adenocarcinomas