J Gastric Cancer.
2014 Dec;14(4):221-228. 10.5230/jgc.2014.14.4.221.
The Value of Postoperative Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Levels for the Early Detection of Gastric Cancer Recurrence after Curative Resection
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. appe98@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2183645
- DOI: http://doi.org/10.5230/jgc.2014.14.4.221
Abstract
- PURPOSE
This study aimed to evaluate the value of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels to detect gastric cancer recurrence.
MATERIALS AND METHODS
We retrospectively reviewed 154 patients who developed recurrence within 2 years after curative gastric cancer surgery and analyzed the relationship between postoperative CEA and CA19-9 levels and recurrence. We readjusted the cut-off values to improve the detection of recurrence. Subgroup analysis according to clinicopathologic variables was performed to further investigate the relationship between recurrence and CEA and CA19-9 levels.
RESULTS
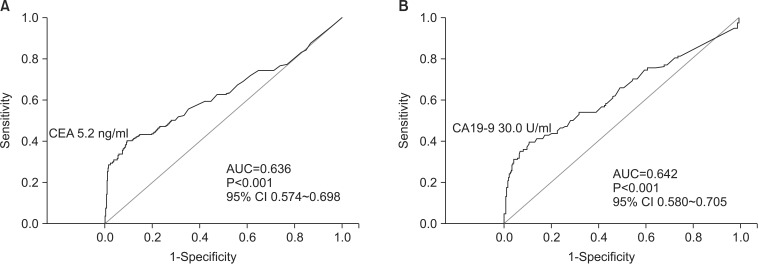
The sensitivity and specificity for elevated CEA levels to detect recurrence were 40.6% and 89.5%, respectively, and those for CA19-9 were 34.2% and 93.6%, respectively. The sensitivity and specificity for elevation of either tumor marker were 54.3% and 84.0%, respectively; those for elevation of both tumor markers were 19.2% and 98.4%, respectively. By readjusting the cut-off values from 5.0 ng/ml to 5.2 ng/ml for CEA and from 37.00 U/ml to 30.0 U/ml for CA19-9, the sensitivity was increased from 34.2% to 40.2% for CA19-9, while there was no increase in sensitivity for CEA. In subgroup analysis, the sensitivity of CEA was higher in patients with elevated preoperative CEA levels than in patients with normal preoperative CEA levels (86.7% versus 33.7%; P<0.001). Furthermore, the sensitivity of CA19-9 was higher in patients with elevated preoperative CA19-9 levels than in patients with normal preoperative CA19-9 levels (82.61% versus 26.83%; P<0.001).
CONCLUSIONS
CEA and/or CA19-9 measurement with the readjusted cut-off values allows for more effective detection of gastric cancer recurrence.
Keyword
MeSH Terms
Figure
Reference
-
1. Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002; 22:2311–2316. PMID: 12174919.2. Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, et al. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997; 20:348–353. PMID: 9256887.
Article3. Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001; 32:41–44. PMID: 11154168.
Article4. Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000; 16:105–110. PMID: 11381189.
Article5. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014; 17:26–33. PMID: 23572188.
Article6. Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001; 181:16–19. PMID: 11248169.
Article7. Marrelli D, Pinto E, De Stefano A, de Manzoni G, Farnetani M, Garosi L, et al. Preoperative positivity of serum tumor markers is a strong predictor of hematogenous recurrence of gastric cancer. J Surg Oncol. 2001; 78:253–258. PMID: 11745820.
Article8. Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, et al. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999; 57:55–62. PMID: 10394126.
Article9. Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001; 22:318–322. PMID: 11553862.
Article10. Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Cianetti A, et al. The role of serum and gastric juice levels of carcinoembryonic antigen, CA199 and CA724 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998; 124:450–455. PMID: 9750022.
Article11. Choi SR, Jang JS, Lee JH, Roh MH, Kim MC, Lee WS, et al. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006; 51:2081–2086. PMID: 17009116.
Article12. Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003; 6:142–145. PMID: 14520526.
Article13. Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer. 2006; 9:74–81. PMID: 16767361.
Article14. Qiu MZ, Lin JZ, Wang ZQ, Wang FH, Pan ZZ, Luo HY, et al. Cutoff value of carcinoembryonic antigen and carbohydrate antigen 19-9 elevation levels for monitoring recurrence in patients with resectable gastric adenocarcinoma. Int J Biol Markers. 2009; 24:258–264. PMID: 20082274.
Article15. Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ, Kim JP, et al. Clinicopathological analysis for recurrence of early gastric cancer. Jpn J Clin Oncol. 2003; 33:209–214. PMID: 12865463.
Article16. D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004; 240:808–816. PMID: 15492562.17. Steinberg WM, Gelfand R, Anderson KK, Glenn J, Kurtzman SH, Sindelar WF, et al. Comparison of the sensitivity and specificity of the CA19-9 and carcinoembryonic antigen assays in detecting cancer of the pancreas. Gastroenterology. 1986; 90:343–349. PMID: 2416628.
Article18. Takasaki H, Uchida E, Tempero MA, Burnett DA, Metzgar RS, Pour PM. Correlative study on expression of CA 19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer patients. Cancer Res. 1988; 48:1435–1438. PMID: 3162196.19. Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978; 299:448–451. PMID: 683276.
Article20. Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010; 17:2321–2329. PMID: 20336387.
Article21. Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002; 15:632–640. PMID: 12065777.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Accuracy of Elevated Serum Carcinoembryonic Antigen for Recurrence in Postoperative Stage II Colorectal Cancer Patients: Comparison With Stage III
- Serum Carcinoembryonic Antigen for Recurrence in Colorectal Cancer Patients
- Postoperative Carcinoembryonic Antigen as a Complementary Tumor Marker of Carbohydrate Antigen 19-9 in Pancreatic Ductal Adenocarcinoma
- Prognostic Factors on Overall Survival in Lymph Node Negative Gastric Cancer Patients Who Underwent Curative Resection
- Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer