J Korean Surg Soc.
2013 Dec;85(6):283-289. 10.4174/jkss.2013.85.6.283.
Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer
- Affiliations
-
- 1Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea. md75769@naver.com
- KMID: 2212551
- DOI: http://doi.org/10.4174/jkss.2013.85.6.283
Abstract
- PURPOSE
The present study is to investigate the clinical utility of tumor marker cutoff ratio (TMR) and develop a TMR combination scoring system based on preoperative tumor marker (TM) levels to prognosis prediction in gastric cancer.
METHODS
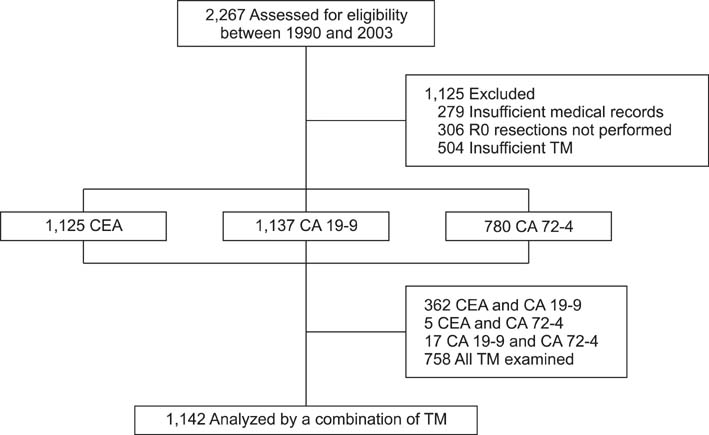
We include 1,142 patients for whom two or more TMs were measured and who underwent radical gastrectomy between 1990 and 2003.
RESULTS
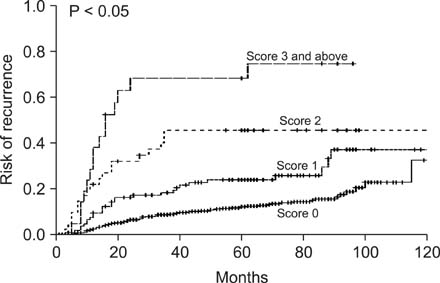
Five-year risk of recurrence (5 YRR) for carcinoembryonic antigen (CEA) TMRs were 18.3%, 29.8%, 61.4% for TMR < 1.0, 1.0 < or = TMR < 2.0, TMR > or = 2.0 respectively. 5 YRR for carbohydrate antigen 19-9 (CA 19-9) TMR were 19.7%, 35.6%, 58.4% for TMR < 1.0, 1.0 < or = TMR < 3.0, TMR > or = 3.0, respectively. 5 YRR for carbohydrate antigen 72-4 (CA 72-4) TMR were 15.2% and 33.6% for TMR < 1.0 and TMR > or = 1.0, respectively. We defined high TMR (TMR > or = 2.0 for CEA, TMR > or = 3.0 for CA19-9), low TMR (1.0 < or = TMR < 2 for CEA, 1.0 < or = TMR < 3.0 for CA 19-9 and 1.0 < or = TMR for CA72-4) and negative TMR (TMR < 1.0 for all TMs). A TMR combination scoring system was devised with negative scored as zero points, low as 1 and high as 2 for each TMR. TMR scores were divided into four categories (score 0, 1, 2, 3 and above) based on the calculated TMR score and 5 YRR were found to be 12.8%, 23.9%, 45.5%, and 68.3%, respectively (P < 0.05). Multivariate analysis showed that our scoring system was a significant independent prognostic factor.
CONCLUSION
Preoperative TMRs such as CEA, CA 19-9, and CA 72-4 show a correlation with prognosis and the TMR combination scoring system could be a useful tool for the prediction of prognosis in gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007; 10:75–83.2. Nam MJ, Oh SJ, Oh CA, Kim DH, Bae YS, Choi MG, et al. Frequency and predictive factors of lymph node metastasis in mucosal cancer. J Gastric Cancer. 2010; 10:162–167.3. Ministry of Health and Welfare. 2002 Annual Report of the Korea Central Cancer Registry. Seoul: Ministry of Health and Welfare;2005.4. Sobin LH, Wittekind CH. International Union Against Cancer (UICC). TNM classification of malignant tumours. 6th ed. New York: John Wiley-Liss;2002.5. Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, et al. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999; 57:55–62.6. Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008; 25:1075–1084.7. Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000; 16:105–110.8. Choi SR, Jang JS, Lee JH, Roh MH, Kim MC, Lee WS, et al. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006; 51:2081–2086.9. Choi KW, Kim HY, Park NG, Kwak NJ, Park KT, Eom JH, et al. Clinical value of serum CA 72-4 as a tumor marker for gastric carcinoma. Korean J Gastroenterol. 1996; 28:164–171.10. Lai IR, Lee WJ, Huang MT, Lin HH. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology. 2002; 49:1157–1160.11. Na KY, Chang YS, Kim YH, Joo SH, Lee SH. The prognostic significance of the preoperative serum CEA, CA19-9 and AFP levels in gastric cancer patients. J Korean Surg Soc. 2008; 75:302–306.12. Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003; 6:142–145.13. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965; 122:467–481.14. Zhou H, Stanners CP, Fuks A. Specificity of anti-carcinoembryonic antigen monoclonal antibodies and their effects on CEA-mediated adhesion. Cancer Res. 1993; 53:3817–3822.15. Baek SW, Cho DH, Yoo CH, Han WK. Prognostic value of preoperative serum CEA and CA19-9 levels in gastric cancer patients. J Korean Surg Soc. 2004; 66:27–32.16. Soeth E, Wirth T, List HJ, Kumbhani S, Petersen A, Neumaier M, et al. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clin Cancer Res. 2001; 7:2022–2030.17. Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997; 9:616–626.18. Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991; 266:14869–14872.19. Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979; 5:957–971.20. Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj J. 2004; 20:353–364.21. Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983; 29:549–552.22. Johnson VG, Schlom J, Paterson AJ, Bennett J, Magnani JL, Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986; 46:850–857.23. Duraker N, Celik AN. The prognostic significance of preoperative serum CA 19-9 in patients with resectable gastric carcinoma: comparison with CEA. J Surg Oncol. 2001; 76:266–271.24. Kim DY, Kim HR, Shim JH, Park CS, Kim SK, Kim YJ. Significance of serum and tissue carcinoembryonic antigen for the prognosis of gastric carcinoma patients. J Surg Oncol. 2000; 74:185–192.25. Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, et al. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000; 3:177–186.26. Park SH, Kim DY, Heo JS, Lim DH, Park CK, Lee KW, et al. Postoperative chemoradiotherapy for gastric cancer. Ann Oncol. 2003; 14:1373–1377.27. Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001; 181:16–19.28. Yasasever V, Sengun Z, Saydan N, Onat H, Dalay N. Serum values of CA72.4 in patients with gastrointestinal system tumors comparison with CEA and CA 19.9. Eur J Gynaecol Oncol. 1992; 13:403–408.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Value of Postoperative Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Levels for the Early Detection of Gastric Cancer Recurrence after Curative Resection
- Postoperative Carcinoembryonic Antigen as a Complementary Tumor Marker of Carbohydrate Antigen 19-9 in Pancreatic Ductal Adenocarcinoma
- The Clinical Relevance of Tumor Marker CA 19-9
- Individualized Cutoff Value of the Serum Carcinoembryonic Antigen Level According to TNM Stage in Colorectal Cancer
- The clinical significance of preoperative serum levels of carbohydrate antigen 19-9 in colorectal cancer