Korean J Physiol Pharmacol.
2017 Mar;21(2):189-195. 10.4196/kjpp.2017.21.2.189.
Comparison of cytotoxicities and wound healing effects of diquafosol tetrasodium and hyaluronic acid on human corneal epithelial cells
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Busan 49241, Korea. jiel75@hanmail.net
- 2Department of Ophthalmology, Gyeongsang National University Changwon Hospital, School of Medicine, Gyeongsang National University, Changwon 51472, Korea.
- 3Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan 50612, Korea.
- KMID: 2371037
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.189
Abstract
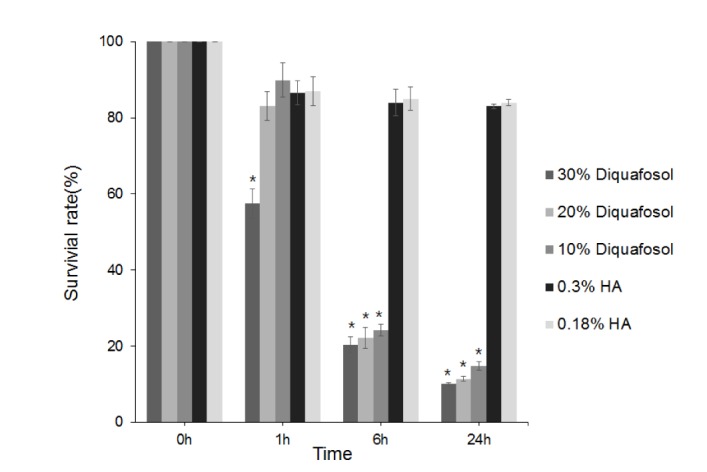
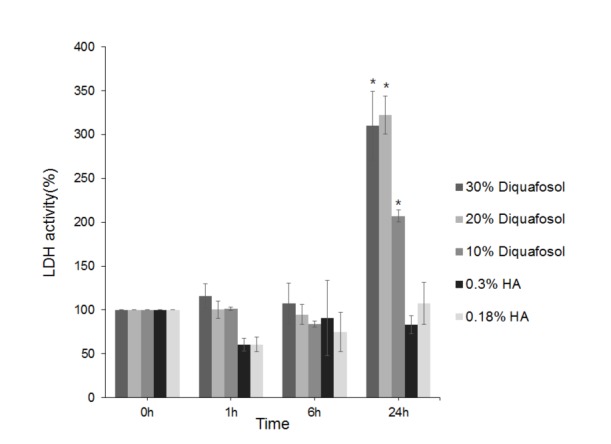
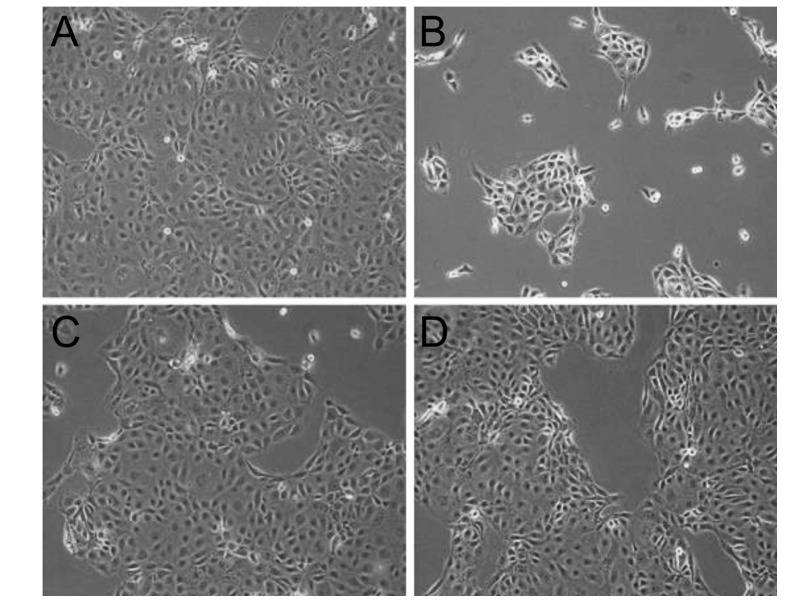
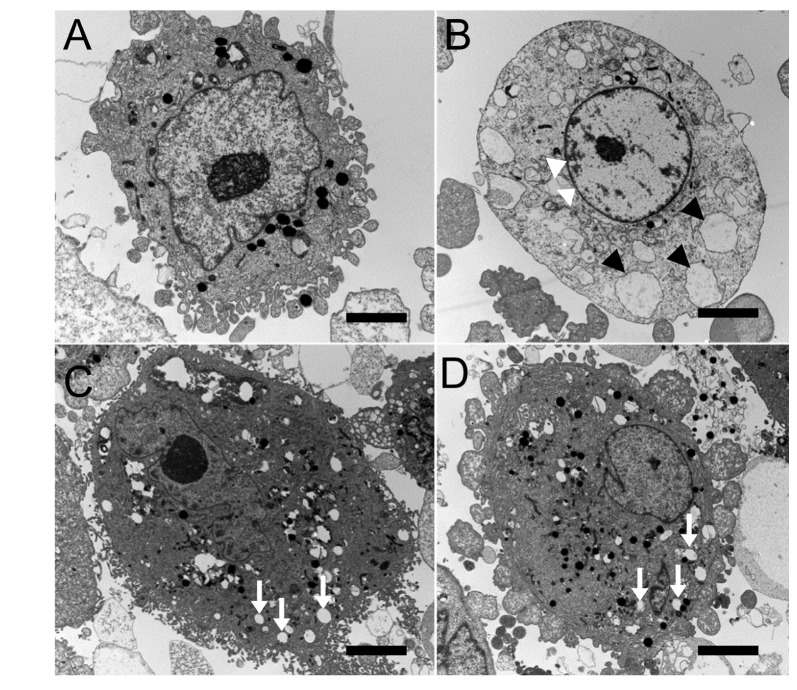
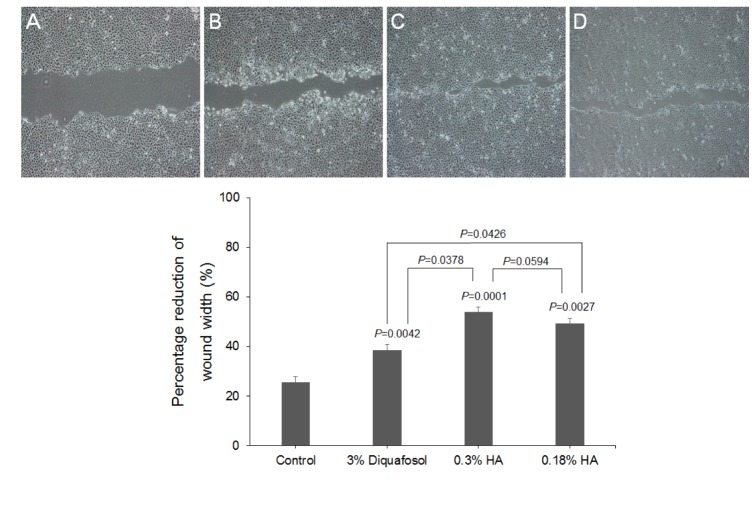
- This study aimed to compare the cellular toxicities of three clinically used dry eye treatments; 3% diquafosol tetrasodium and hyaluronic acid at 0.3 and 0.18%. A methyl thiazolyltetrazoiun (MTT)-based calorimetric assay was used to assess cellular proliferation and a lactate dehydrogenase (LDH) leakage assay to assess cytotoxicity, using Human corneal epithelial cells (HCECs) exposed to 3% diquafosol tetrasodium, 0.3% hyaluronic acid (HA), or 0.18% HA or 1, 6 or 24 h. Cellular morphology was evaluated by inverted phase-contrast light microscopy and electron microscopy, and wound widths were measured 24 h after confluent HCECs were scratched. Diquafosol had a significant, time-dependent, inhibitory effect on HCEC proliferation and cytotoxicity. HCECs treated with diquafosol detached more from the bottoms of dishes and damaged cells showed degenerative changes, such as, reduced numbers of microvilli, vacuole formation, and chromatin of the nuclear remnant condensed along the nuclear periphery. All significantly stimulated reepithelialization of HCECs scratched, which were less observed in diquafosol. Therefore, epithelial toxicity should be considered after long-term usage of diquafosol and in overdose cases, especially in dry eye patients with pre-existing punctated epithelial erosion.
Keyword
MeSH Terms
Figure
Reference
-
1. Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, Dogru M, Schaumberg DA, Kawakita T, Takebayashi T, Tsubota K. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011; 118:2361–2367. PMID: 21889799.
Article2. Lee JE, Kim NM, Yang JW, Kim SJ, Lee JS, Lee JE. A randomised controlled trial comparing a thermal massager with artificial tear-drops for the treatment of dry eye. Br J Ophthalmol. 2014; 98:46–51. PMID: 24133024.
Article3. Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004; 88:821–825. PMID: 15148219.
Article4. Yamaguchi M, Tsubota K, Watanabe H, Ohashi Y. The safety and efficacy of long-term treatment with 3% diquafosol ophthalmic solution for dry eye. Atarashii Ganka (J Eye). 2012; 29:527–535.5. Takaoka-Shichijo Y, Sakamoto A, Nakamura M. Effect of diquafosol tetrasodium on MUC5AC secretion by rabbit conjunctival tissues. Atarashii Ganka (J Eye). 2011; 28:261–265.6. Li Y, Kuang K, Yerxa B, Wen Q, Rosskothen H, Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl− and fluid secretion. Am J Physiol Cell Physiol. 2001; 281:C595–C602. PMID: 11443059.7. Lee JE, Sun Y, Gjorstrup P, Pearlman E. Inhibition of corneal inflammation by the resolvin E1. Invest Ophthalmol Vis Sci. 2015; 56:2728–2736. PMID: 25758817.
Article8. Arita R, Suehiro J, Haraguchi T, Maeda S, Maeda K, Tokoro H, Amano S. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013; 97:725–729. PMID: 23584719.
Article9. Lee JS, Lee SU, Che CY, Lee JE. Comparison of cytotoxicity and wound healing effect of carboxymethylcellulose and hyaluronic acid on human corneal epithelial cells. Int J Ophthalmol. 2015; 8:215–221. PMID: 25938030.10. Lee JS, Lee JE, Kim N, Oum BS. Comparison of the conjunctival toxicity of topical ocular antiallergic agents. J Ocul Pharmacol Ther. 2008; 24:557–562. PMID: 19049267.
Article11. Park YS. Physiology of body fluid. In : Kang DH, editor. Physiology. 5th ed. Seoul: Sin-Kwang;2000. p. 585–606.12. Garrett Q, Khandekar N, Shih S, Flanagan JL, Simmons P, Vehige J, Willcox MD. Betaine stabilizes cell volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress. Exp Eye Res. 2013; 108:33–41. PMID: 23246691.
Article13. Kim K. Acid-base balance. In : Sung H, Kim KH, editors. Physiology. 3rd ed. Seoul: Eui-hak;2006. p. 326–327.14. Ayaki M, Iwasawa A, Yaguchi S, Koide R. In vitro assessment of the cytotoxicity of anti-allergic eye drops using 5 cultured corneal and conjunctival cell lines. J Oleo Sci. 2011; 60:139–144. PMID: 21343662.15. Pauly A, Brasnu E, Riancho L, Brignole-Baudouin F, Baudouin C. Multiple endpoint analysis of BAC-preserved and unpreserved antiallergic eye drops on a 3D-reconstituted corneal epithelial model. Mol Vis. 2011; 17:745–755. PMID: 21437201.16. Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM, Warnet JM, Baudouin C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008; 33:303–312. PMID: 18398704.17. De Saint Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr Eye Res. 2000; 20:85–94. PMID: 10617908.
Article18. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29:312–334. PMID: 20302969.
Article19. Champeau EJ, Edelhauser HF. The effect of ophthalmic preservatives on the ocular surface: conjunctival and corneal uptake and distribution of Benzalkonium chloride and chlorhexidine digluconate. In : Holly FJ, editor. The preocular tear film in health, disease and contact lens wear. Lubbock, TX: Dry eye Institute, Inc.;1986.20. Dutot M, Pouzaud F, Larosche I, Brignole-Baudouin F, Warnet JM, Rat P. Fluoroquinolone eye drop-induced cytotoxicity: role of preservative in P2X7 cell death receptor activation and apoptosis. Invest Ophthalmol Vis Sci. 2006; 47:2812–2819. PMID: 16799018.
Article21. Feng MM, Baryla J, Liu H, Laurie GW, McKown RL, Ashki N, Bhayana D, Hutnik CM. Cytoprotective effect of lacritin on human corneal epithelial cells exposed to benzalkonium chloride in vitro. Curr Eye Res. 2014; 39:604–610. PMID: 24401093.22. Guzman-Aranguez A, Calvo P, Ropero I, Pintor J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. J Ocul Pharmacol Ther. 2014; 30:790–798. PMID: 25100331.23. Byun YS, Yoo YS, Kwon JY, Joo JS, Lim SA, Whang WJ, Mok JW, Choi JS, Joo CK. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp Eye Res. 2016; 143:89–97. PMID: 26505315.
Article24. Mohamed YH, Uematsu M, Ueki R, Inoue D, Fujikawa A, Sasaki H, Kitaoka T. Acute Corneal Toxicity of Diquas. Pharmacology. 2016; 98:56–61. PMID: 27078164.
Article25. Lazarus HM, Imperia PS, Botti RE, Mack RJ, Lass JH. An in vitro method which assesses corneal epithelial toxicity due to antineoplastic, preservative and antimicrobial agents. Lens Eye Toxic Res. 1989; 6:59–85. PMID: 2488034.26. Tripathi BJ, Tripathi RC. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res. 1989; 6:395–403. PMID: 2486935.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hyaluronic Acid and Epidermal Growth Factor on Cultured Corneal Epithelial Cells in the Rabbit
- The Comparison of the Corneal Epithelial Healing According to Treatment Modalities in Rabbit
- Clinical Efficacy of Topical 3% Diquafosol Tetrasodium in Short Tear Film Break-Up Time Dry Eye
- The Effect of Topically Applied Sodium-hyaluronic acid(Na-HA) on Corneal Epithelial Healing
- Clinical Efficacy of Topical Diquafosol Tetrasodium after Laser Epithelial Keratomileusis