Intest Res.
2017 Jan;15(1):68-74. 10.5217/ir.2017.15.1.68.
Single fecal microbiota transplantation failed to change intestinal microbiota and had limited effectiveness against ulcerative colitis in Japanese patients
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. takagast@keio.jp
- 2Department of Immunology, Keio University School of Medicine, Tokyo, Japan.
- 3Laboratory of Metagenomics, Graduate School of Frontier Sciences, The University of Tokyo, Chiba, Japan.
- 4Graduate School of Advanced Science and Engineering, Waseda University, Tokyo, Japan.
- KMID: 2368487
- DOI: http://doi.org/10.5217/ir.2017.15.1.68
Abstract
- BACKGROUND/AIMS
Recent developments in analytical techniques including next-generation sequencing have clarified the correlation between intestinal microbiota and inflammatory bowel disease. Fecal microbiota transplantation (FMT) for patients with ulcerative colitis (UC) is proposed as a potential approach to resolving their dysbiosis; however, its safety and efficacy have not been confirmed. This single-arm, open-label, non-randomized study aimed to evaluate the safety and efficacy of FMT for Japanese patients with UC as the first registered clinical trial in Japan.
METHODS
We enrolled 10 patients with active UC despite medical therapy. The donors were the patients' relatives and were carefully screened for infectious diseases. Fecal material was administered via colonoscopy, and the primary endpoint was the presence or absence of serious adverse events related to FMT. The secondary endpoint was a change in partial Mayo score at 12 weeks post-FMT. Scores ≤2 were considered a clinical response. Fecal samples were collected to follow changes in gut microbiota, while extracted complementary DNA were analyzed by a next-generation sequencer. We obtained written informed consent from all patients and donors. This study was approved by our Institutional Review Board and is registered in the University hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN 000012814).
RESULTS
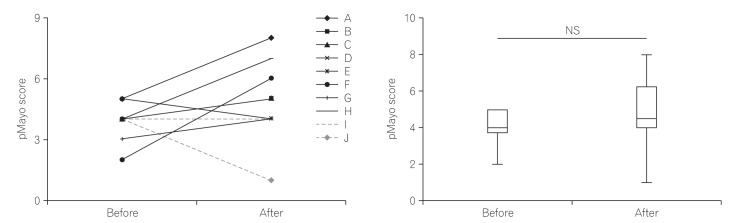
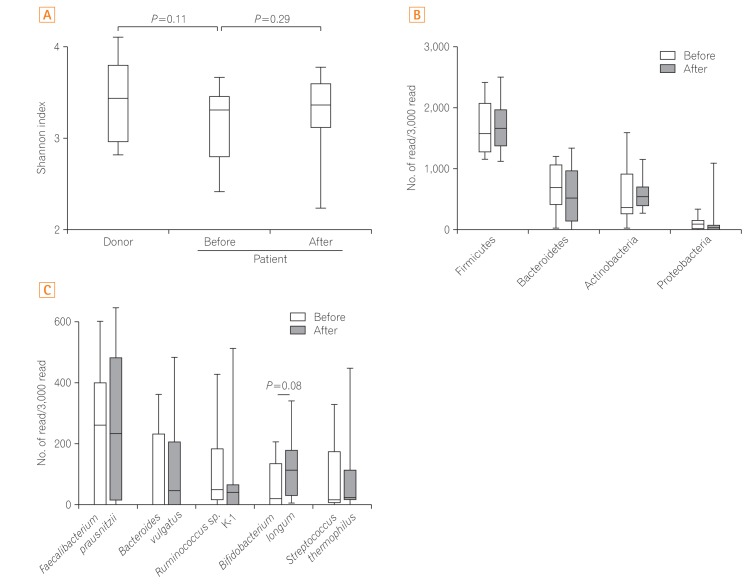
Five patients with moderate disease and five with severe disease were enrolled. No severe adverse effects were observed. One patient achieved clinical response; however, none of the patients' microbiota diversity recovered to the donor levels.
CONCLUSIONS
The use of single FMT for UC was safe; however, we failed to show its clinical efficacy and potential to change the intestinal microbiota.
MeSH Terms
-
Asian Continental Ancestry Group*
Colitis, Ulcerative*
Colonoscopy
Communicable Diseases
DNA, Complementary
Dysbiosis
Ethics Committees, Research
Fecal Microbiota Transplantation*
Gastrointestinal Microbiome*
Humans
Inflammatory Bowel Diseases
Information Services
Informed Consent
Japan
Microbiota
Tissue Donors
Treatment Outcome
Ulcer*
DNA, Complementary
Figure
Cited by 2 articles
-
Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
Young-Seok Cho
Intest Res. 2019;17(1):6-8. doi: 10.5217/ir.2018.00171.Is there a potential role of fecal microbiota transplantation in the treatment of inflammatory bowel disease?
Chang Soo Eun
Intest Res. 2017;15(2):145-146. doi: 10.5217/ir.2017.15.2.145.
Reference
-
1. Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016; 14:127–138. PMID: 27175113.
Article2. Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012; 107:1452–1459. PMID: 23034604.
Article3. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010; 28:573–621. PMID: 20192811.
Article4. Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007; 131:33–45. PMID: 17923086.
Article5. Takaishi H, Matsuki T, Nakazawa A, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008; 298:463–472. PMID: 17897884.
Article6. Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011; 145:745–757. PMID: 21565393.
Article7. Chen WX, Ren LH, Shi RH. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J Gastroenterol. 2014; 20:15657–15663. PMID: 25400449.
Article8. Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006; 24(Suppl 3):90–95. PMID: 16961752.
Article9. Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007; (4):CD005573. DOI: 10.1002/14651858.CD005573.pub2. PMID: 17943867.
Article10. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368:407–415. PMID: 23323867.
Article11. Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013; 108:1620–1630. PMID: 24060759.
Article12. Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013; 19:2155–2165. PMID: 23899544.
Article13. Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol. 2013; 27:127–137. PMID: 23768558.
Article14. Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014; 5:e00893–e00914. DOI: 10.1128/mBio.00893-14. PMID: 24939885.
Article15. Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G310–G319. PMID: 24284963.16. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014; 8:1569–1581. PMID: 25223604.
Article17. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.e6. PMID: 25857665.
Article18. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118.e4. PMID: 25836986.
Article19. Nishijima S, Suda W, Oshima K, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016; 23:125–133. PMID: 26951067.
Article20. Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007; 14:169–181. PMID: 17916580.
Article21. Matsuoka K, Mizuno S, Hayashi A, Hisamatsu T, Naganuma M, Kanai T. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014; 63:69–74. PMID: 25500625.
Article22. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011; 5:169–172. PMID: 20827291.
Article23. Sugahara H, Odamaki T, Fukuda S, et al. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep. 2015; 5:13548. DOI: 10.1038/srep13548. PMID: 26315217.
Article24. Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013; 145:396–406.e10. PMID: 23665276.
Article25. Masui R, Sasaki M, Funaki Y, et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis. 2013; 19:2848–2856. PMID: 24141712.
Article26. Miyauchi E, Ogita T, Miyamoto J, et al. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PLoS One. 2013; 8:e79735. DOI: 10.1371/journal.pone.0079735. PMID: 24255712.
Article27. Srutkova D, Schwarzer M, Hudcovic T, et al. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute DSS-induced colitis in strictly strain-specific manner. PLoS One. 2015; 10:e0134050. DOI: 10.1371/journal.pone.0134050. PMID: 26218526.
Article28. Wei P, Yang Y, Ding Q, et al. Oral delivery of Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to combat ulcerative colitis. J Med Microbiol. 2016; 65:160–168. PMID: 26567174.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
- Fecal microbiota transplantation for recurrent Clostridium difficile infection in a patient with ulcerative colitis
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases
- Fecal Microbiota Transplantation and Its Usage in Neuropsychiatric Disorders
- Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis?