World J Mens Health.
2016 Dec;34(3):209-216. 10.5534/wjmh.2016.34.3.209.
Effects of Betulinic Acid on the Male Reproductive System of a Streptozotocin-Nicotinamide-Induced Diabetic Mouse Model
- Affiliations
-
- 1Health Research Institute, Diabetes Research Center, Department of Physiology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 2Department of Physiology, Student Research Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. aliakbar_oroojan@yahoo.com
- 3Cell and Molecular Research Center, Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 4Golestan Hospital Clinical Research Development Unit, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- KMID: 2364204
- DOI: http://doi.org/10.5534/wjmh.2016.34.3.209
Abstract
- PURPOSE
The present study was conducted to evaluate the favorable or harmful effects of betulinic acid (BA) on a diabetic reproductive system.
MATERIALS AND METHODS
In this experimental study, 60 male Naval Medical Research Institute mice (20∼25 g) were randomly divided into 6 groups: control, diabetes, diabetes+BA (10, 20, and 40 mg/kg), and diabetes+ metformin (200 mg/kg). A diabetic model was induced by a single dose of streptozotocin (STZ) (65 mg/kg) injection intraperitoneally 15 minutes after an intraperitoneal administration of nicotinamide (NA) (120 mg/kg). BA and metformin were gavaged for 2 weeks after confirmed diabetes induction in the treatment groups. One day after the last treatment, plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone levels were evaluated. The cauda epididymis and testis were removed to analyze the sperm count and testis histopathology.
RESULTS
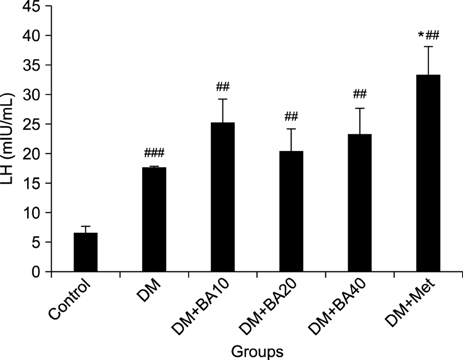
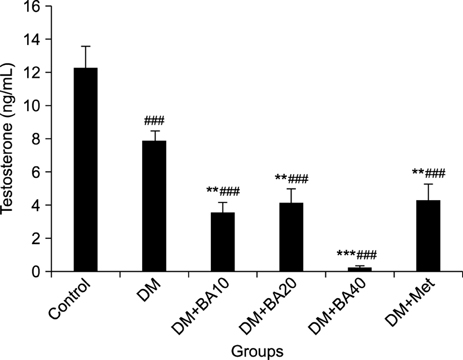
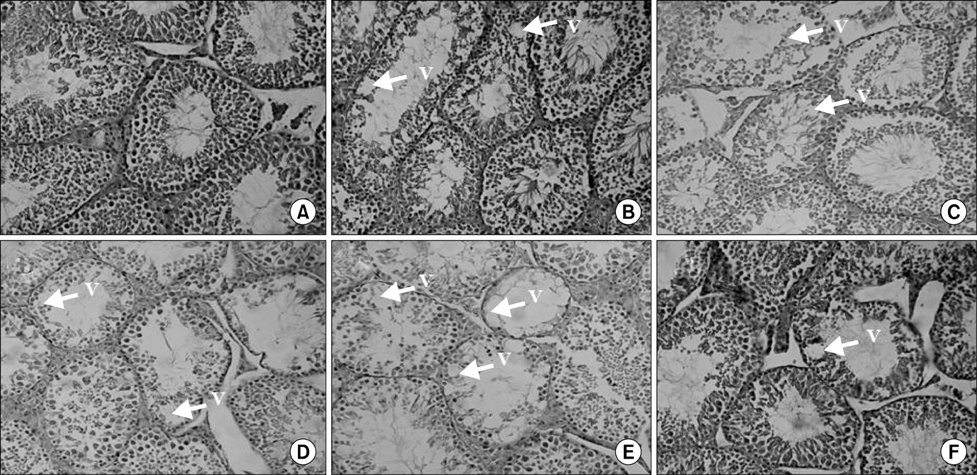
LH levels increased in diabetic (p<0.001) and diabetic BA-treated mice (p=0.009). Plasma levels of testosterone (p< 0.001) and sperm count (p=0.04) decreased in these groups when compared to the control group. Furthermore, administration of 10 mg/kg (p=0.001), 20 mg/kg (p=0.004), or 40 mg/kg (p<0.001) of BA led to a greater reduction in plasma testosterone levels compared to the diabetes group. Seminiferous tubule vacuole numbers increased in diabetic and diabetic BA-treated mice, but testis morphology and FSH level assessment revealed no significant differences between the groups.
CONCLUSIONS
STZ-NA can induce diabetic alterations in the male reproductive system and the administration of BA in diabetic treated mice resulted in a worse outcome.
Keyword
MeSH Terms
-
Academies and Institutes
Animals
Diabetes Mellitus
Epididymis
Follicle Stimulating Hormone
Humans
Luteinizing Hormone
Male*
Metformin
Mice*
Niacinamide
Plasma
Seminiferous Tubules
Sperm Count
Spermatozoa
Streptozocin
Testis
Testosterone
Vacuoles
Follicle Stimulating Hormone
Luteinizing Hormone
Metformin
Niacinamide
Streptozocin
Testosterone
Figure
Reference
-
1. Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 2015; 17:948–953.
Article2. Mulholland J, Mallidis C, Agbaje I, McClure N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online. 2011; 22:215–219.
Article3. Zheng R, Cao L, Cao W, Chu X, Hu Y, Zhang H, et al. Risk factors for hypogonadism in male patients with type 2 diabetes. J Diabetes Res. 2016; 2016:5162167.
Article4. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012; 33:145–153.
Article5. Ahangarpour A, Oroojan AA, Heidari H, Ehsan G, Rashidi Nooshabadi MR. Effects of hydro-alcoholic extract of Rhus coriaria (Sumac) seeds on reproductive complications of nicotinamide-streptozotocin induced type-2 diabetes in male mice. World J Mens Health. 2014; 32:151–158.6. Csuk R. Betulinic acid and its derivatives: a patent review (2008-2013). Expert Opin Ther Pat. 2014; 24:913–923.7. Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005; 12:657–666.
Article8. Ko BS, Kang S, Moon BR, Ryuk JA, Park S. A 70% ethanol extract of mistletoe rich in Betulin, betulinic acid, and Oleanolic acid potentiated β-cell function and mass and enhanced hepatic insulin sensitivity. Evid Based Complement Alternat Med. 2016; 2016:7836823.9. Ahangarpour A, Oroojan AA, Heydari H. Effect of hydro-alcoholic extract of dorema aucheri on serum levels of testosterone, FSH and sperm count in nicotinamide-STZ-induced diabetic rat models. ZUMS J. 2013; 21:22–31.10. Khanra R, Dewanjee S, K Dua T, Sahu R, Gangopadhyay M, De Feo V, et al. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015; 13:6.
Article11. Oyebanji BO, Saba AB, Oridupa OA. Studies on the anti-inflammatory, analgesic and antipyrexic activities of betulinic acid derived from Tetracera potatoria. Afr J Tradit Complement Altern Med. 2013; 11:30–33.
Article12. Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of type 2 diabetic embryopathy. Diabetes. 2015; 64:2526–2536.
Article13. Ahangarpour A, Oroojan AA, Heidari H. Effects of exendin-4 on male reproductive parameters of d-galactose induced aging mouse model. World J Mens Health. 2014; 32:176–183.
Article14. Ahangarpour A, Oroojan AA, Aliakbari FR. Effects of C-peptide and nicotinamide on serum LH, FSH, testosterone levels and sperm count in nicotinamide/streptozotocin-induced-diabetes in mice. Acta Endo (Buc). 2014; 10:588–594.15. Talebi AR, Khorsandi L, Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet. 2013; 30:1203–1209.
Article16. Aritajat S, Wutteerapol S, Saenphet K. Anti-diabetic effect of Thunbergia laurifolia Linn. aqueous extract. Southeast Asian J Trop Med Public Health. 2004; 35:Suppl 2. 53–58.17. Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol. 2004; 6:Suppl 6. S3–S8.18. Asare-Anane H, Ofori EK, Yeboah FA, Tagoe EA, Bani SB, Bawah AT, et al. Primary hypogonadism in Ghanaian men with type 2 diabetes mellitus. Int J Sci Technol Res. 2013; 2:310–315.19. Liu CY, Hsu YJ, Chien YW, Cha TL, Tsao CW. Dietary resistant maltodextrin ameliorates testicular function and spermatogenesis in streptozotocin-nicotinamide-induced diabetic rats. Andrologia. 2016; 48:363–373.
Article20. Rato L, Alves MG, Dias TR, Cavaco JE, Oliveira PF. Testicular metabolic reprogramming in neonatal streptozotocin-induced type 2 diabetic rats impairs glycolytic flux and promotes glycogen synthesis. J Diabetes Res. 2015; 2015:973142.
Article21. Butchi Akondi R, Kumar P, Annapurna A, Pujari M. Protective effect of rutin and naringin on Sperm Quality in Streptozotocin (STZ) induced type 1 diabetic rats. Iran J Pharm Res. 2011; 10:585–596.22. Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med. 2014; 11:1–8.
Article23. Dathe S, Paasch U, Grunewald S, Glander HJ. Mitochondrial damage in human sperm caused by the antineoplastic agent betulinic acid. Hautarzt. 2005; 56:768–772.24. Prasanna Raja P, Sivakumar V, Riyazullah MS. Antidiabetic potential of aqueous and ethanol leaf extracts of vitex negundo. Int J Pharmacogn Phytochem Res. 2012; 4:38–40.25. Suhasini S, Elanchezhiyan C, Babby A. Hepatoprotective effect of Ipomoea pes-caprae leaves extract in streptozotocin induced diabetic rats. Int J Pharm Pharm Sci Res. 2014; 4:27–30.26. Al-Yahya AA. Reproductive toxicity of orthosiphon stamineus Benth (java tea) in Swiss albino mice. Br J Pharmacol Toxicol. 2013; 4:181–187.27. Achard C, Courtillot C, Lahuna O, Méduri G, Soufir JC, Lière P, et al. Normal spermatogenesis in a man with mutant luteinizing hormone. N Engl J Med. 2009; 361:1856–1863.
Article28. Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol. 2001; 29:64–76.
Article29. Trindade AA, Simões AC, Silva RJ, Macedo CS, Spadella CT. Long term evaluation of morphometric and ultrastructural changes of testes of alloxan-induced diabetic rats. Acta Cir Bras. 2013; 28:256–265.
Article30. Vidal JD, Whitney KM. Morphologic manifestations of testicular and epididymal toxicity. Spermatogenesis. 2014; 4:e979099.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Hydro-Alcoholic Extract of Rhus coriaria (Sumac) Seeds on Reproductive Complications of Nicotinamide-Streptozotocin Induced Type-2 Diabetes in Male Mice
- Effects of Myricitrin and Solid Lipid NanoparticleContaining Myricitrin on Reproductive System Disorders Induced by Diabetes in Male Mouse
- The Role of the Central Parasympathetic Nervous System in Modulating Glucose Metabolism in Streptozotocin-induced Diabetic Rats
- The Effects of Insulin Treatment on the Contractile Responses of the Seminal Vesicle in Streptozotocin-induced Diabetic Rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats