Cancer Res Treat.
2016 Oct;48(4):1229-1242. 10.4143/crt.2015.500.
A New Cell Block Method for Multiple Immunohistochemical Analysis of Circulating Tumor Cells in Patients with Liver Cancer
- Affiliations
-
- 1Department of Pathology, Research Institute and Hospital, National Cancer Center, Goyang, Korea. jwpark@ncc.re.kr
- 2Colorectal Cancer Branch, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 3Center for Liver Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. heejincmd@ncc.re.kr
- KMID: 2356225
- DOI: http://doi.org/10.4143/crt.2015.500
Abstract
- PURPOSE
We developed a new method of detecting circulating tumor cells (CTCs) in liver cancer patients by constructing cell blocks from peripheral blood cells, including CTCs, followed by multiple immunohistochemical analysis.
MATERIALS AND METHODS
Cell blockswere constructed from the nucleated cell pellets of peripheral blood afterremoval of red blood cells. The blood cell blocks were obtained from 29 patients with liver cancer, and from healthy donor blood spikedwith seven cell lines. The cell blocks and corresponding tumor tissues were immunostained with antibodies to seven markers: cytokeratin (CK), epithelial cell adhesion molecule (EpCAM), epithelial membrane antigen (EMA), CK18, α-fetoprotein (AFP), Glypican 3, and HepPar1.
RESULTS
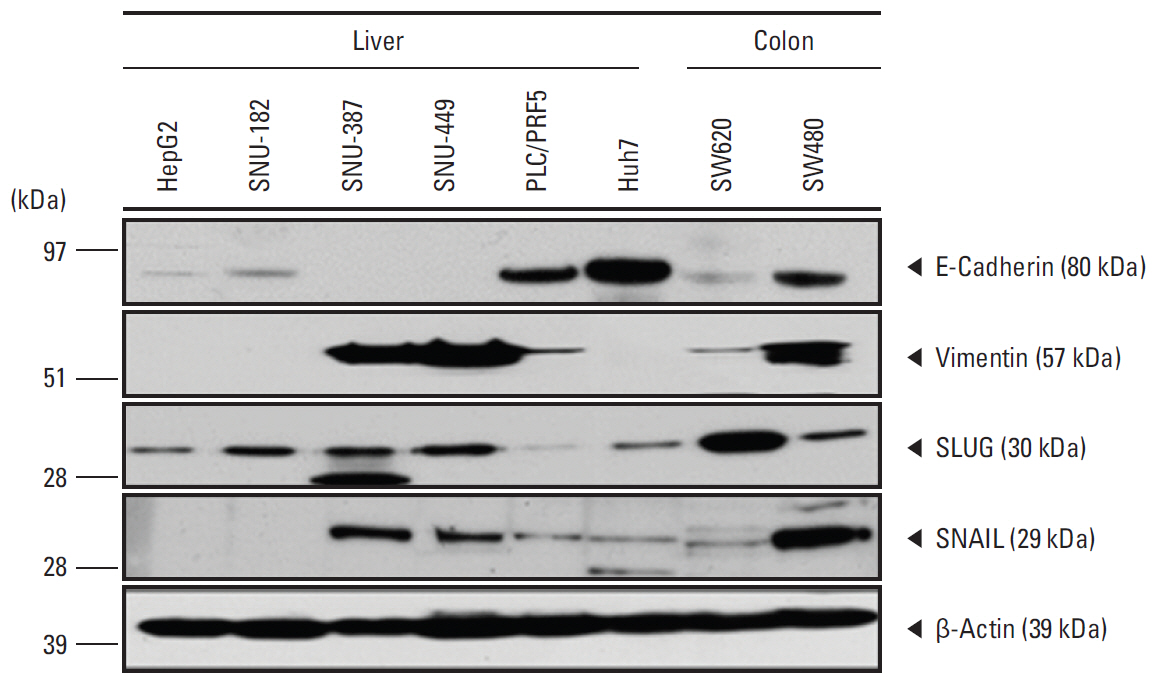
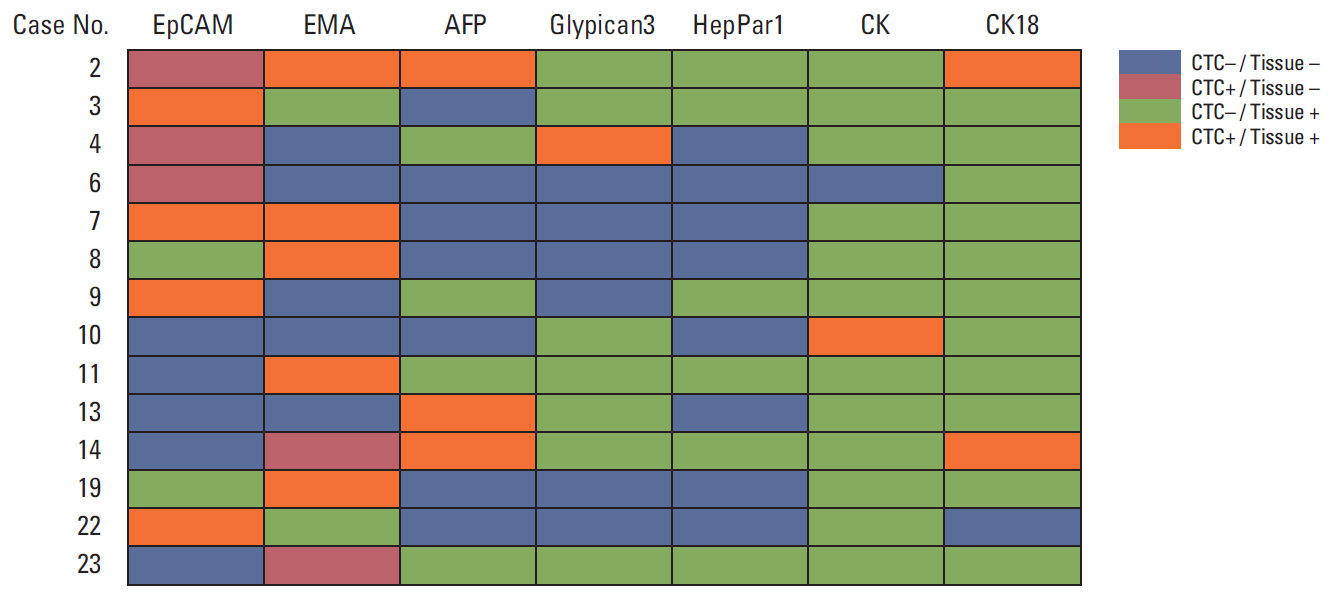
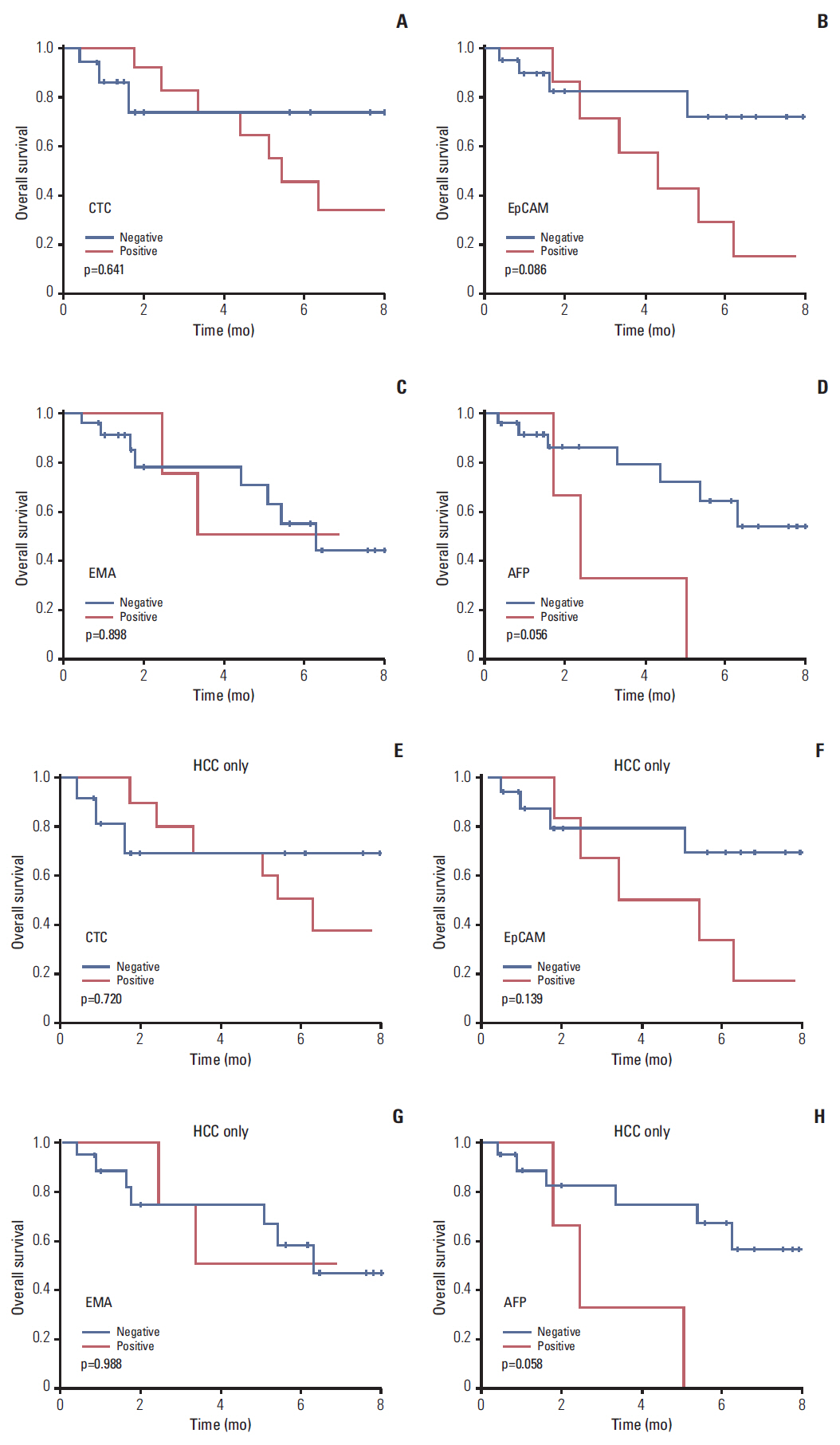
The average recovery rate of spiked SW620 cells from blood cell blocks was 91%. CTCs were detected in 14 out of 29 patients (48.3%); 11/23 hepatocellular carcinomas (HCC), 1/2 cholangiocarcinomas (CC), 1/1 combined HCC-CC, and 1/3 metastatic cancers. CTCs from 14 patients were positive for EpCAM (57.1%), EMA (42.9%), AFP (21.4%), CK18 (14.3%), Gypican3 and CK (7.1%, each), and HepPar1 (0%). Patients with HCC expressed EpCAM, EMA, CK18, and AFP in tissue and/or CTCs, whereas CK, HepPar1, and Glypican3 were expressed only in tissue. Only EMA was significantly associated with the expressions in CTC and tissue. CTC detection was associated with higher T stage and portal vein invasion in HCC patients.
CONCLUSION
This cell block method allows cytologic detection and multiple immunohistochemical analysis of CTCs. Our results show that tissue biomarkers of HCC may not be useful for the detection of CTC. EpCAM could be a candidate marker for CTCs in patients with HCC.
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015; 35:2155–66.3. Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014; 21:147–54.
Article4. Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014; 21:1002–9.
Article5. Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014; 141:209–21.
Article6. Hyun KA, Kwon K, Han H, Kim SI, Jung HI. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens Bioelectron. 2013; 40:206–12.
Article7. Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014; 25:1506–16.
Article8. Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012; 38:68–75.
Article9. Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007; 450:1235–9.
Article10. Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004; 35:122–8.
Article11. Park H, Lee H, Seo AN, Cho JY, Choi YR, Yoon YS, et al. SALL4 expression in hepatocellular carcinomas is associated with EpCAM-positivity and a poor prognosis. J Pathol Transl Med. 2015; 49:373–81.
Article12. Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, et al. Validation of a model of colon cancer progression. J Pathol. 2000; 192:446–54.
Article13. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010.14. Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. 2nd ed. New York: Springer;2012.15. Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000; 156:57–63.16. Zieglschmid V, Hollmann C, Bocher O. Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci. 2005; 42:155–96.
Article17. Rolle A, Gunzel R, Pachmann U, Willen B, Hoffken K, Pachmann K. Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: a preliminary report. World J Surg Oncol. 2005; 3:18.18. Chiappini F. Circulating tumor cells measurements in hepatocellular carcinoma. Int J Hepatol. 2012; 2012:684802.
Article19. Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011; 17:3783–93.
Article20. Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011; 254:569–76.21. Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015; 21:4786–800.
Article22. Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013; 133:2165–71.
Article23. Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013; 57:1458–68.
Article24. Ruck P, Wichert G, Handgretinger R, Kaiserling E. Ep-CAM in malignant liver tumours. J Pathol. 2000; 191:102–3.
Article25. de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999; 188:201–6.
Article26. Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumorinitiating cells with stem/progenitor cell features. Gastroenterology. 2009; 136:1012–24.
Article27. Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010; 52:280–1.
Article28. Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. 2012; 31:673–87.
Article29. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133:704–15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translational Application of Single-cell Transcriptomic Analysis in Hepatocellular Carcinoma
- Role of Liquid Biopsies in Colorectal Cancer
- Circulating Tumor Cells and Extracellular Nucleic Acids in Breast Cancer
- Clinical Application of Circulating Tumor DNA Analysis
- Circulating Tumor Cell and Cell-free Circulating Tumor DNA in Lung Cancer