Korean Circ J.
2016 Nov;46(6):753-761. 10.4070/kcj.2016.46.6.753.
The Roles of CD137 Signaling in Atherosclerosis
- Affiliations
-
- 1Department of Life Sciences, Ewha Womans University, Seoul, Korea. gootaeg@ewha.ac.kr
- 2Cardiovascular Division, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
- KMID: 2355448
- DOI: http://doi.org/10.4070/kcj.2016.46.6.753
Abstract
- The tumor necrosis factor receptor superfamily (TNFRSF), which includes CD40, LIGHT, and OX40, plays important roles in the initiation and progression of cardiovascular diseases, involving atherosclerosis. CD137, a member of TNFRSF, is a well-known activation-induced T cell co-stimulatory molecule and has been reported to be expressed in human atherosclerotic plaque lesions, and plays pivotal roles in mediating disease processes. In this review, we focus on and summarize recent advances in mouse studies on the involvement of CD137 signaling in the pathogenesis and plaque stability of atherosclerosis, thereby highlighting a valuable therapeutic target in atherosclerosis.
Keyword
MeSH Terms
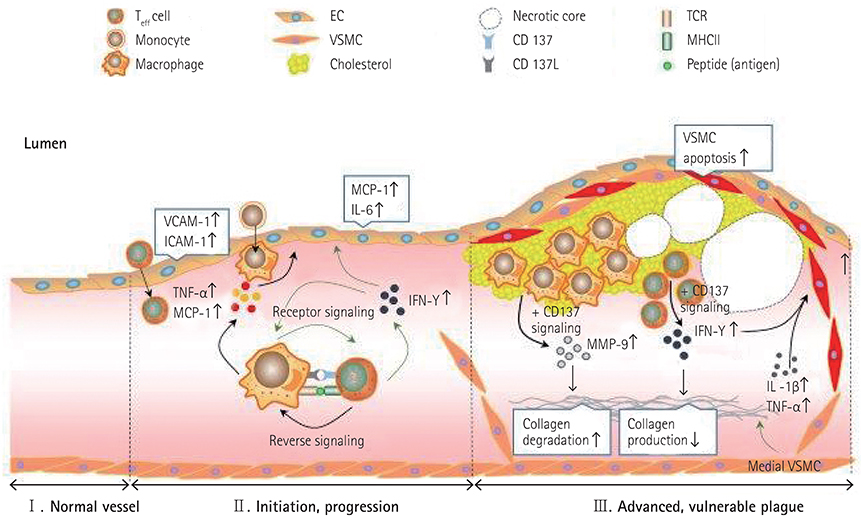
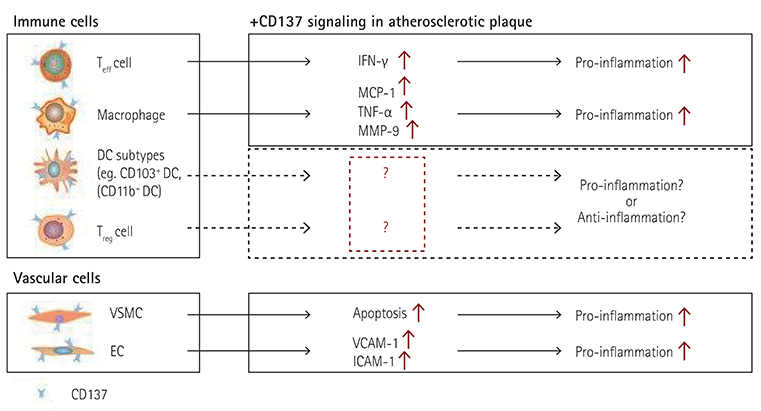
Figure
Reference
-
1. Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002; 8:1218–1226.2. Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002; 8:1211–1217.3. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; (3):204–212.4. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; (7):508–519.5. Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002; 91:281–291.6. Stancel N, Chen CC, Ke LY, et al. Interplay between CRP, Atherogenic LDL, and LOX-1 and Its Potential Role in the Pathogenesis of Atherosclerosis. Clin Chem. 2016; 62:320–327.7. Björkbacka H, Fredrikson GN, Nilsson J. Emerging biomarkers and intervention targets for immune-modulation of atherosclerosis - a review of the experimental evidence. Atherosclerosis. 2013; 227:9–17.8. Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006; 26:531–535.9. Tabas I, Tall A, Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ Res. 2010; 106:58–67.10. Lusis AJ. Atherosclerosis. Nature. 2000; 407:233–241.11. Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med. 2007; 17:253–258.12. Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012; 11:1062–1070.13. Zhang GB, Dong QM, Hou JQ, et al. Characterization and application of three novel monoclonal antibodies against human 4-1BB: distinct epitopes of human 4-1BB on lung tumor cells and immune cells. Tissue Antigens. 2007; 70:470–479.14. Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol. 2001; 115:543–549.15. Wan YL, Zheng SS, Zhao ZC, Li MW, Jia CK, Zhang H. Expression of co-stimulator 4-1BB molecule in hepatocellular carcinoma and adjacent non-tumor liver tissue, and its possible role in tumor immunity. World J Gastroenterol. 2004; 10:195–199.16. Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006; 1:297–329.17. Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006; 86:515–581.18. Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002; 27:19–26.19. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001; 104:487–501.20. Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006; 26:2421–2432.21. Kwon B, Kim BS, Cho HR, Park JE, Kwon BS. Involvement of tumor necrosis factor receptor superfamily(TNFRSF) members in the pathogenesis of inflammatory diseases. Exp Mol Med. 2003; 35:8–16.22. Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006; 177:4464–4472.23. Binder CJ, Hartvigsen K, Chang MK, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004; 114:427–437.24. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016; 20:17–28.25. Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010; 121:1746–1755.26. Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012; 110:675–687.27. Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009; 206:2067–2077.28. Liao YH, Xia N, Zhou SF, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012; 59:420–429.29. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133:775–787.30. Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008; 9:239–244.31. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008; 8:523–532.32. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003; 4:330–336.33. Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007; 8:457–462.34. Foks AC, Litchman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015; 35:280–287.35. Mor A, Planer D, Luboshits G, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007; 27:893–900.36. Wang Z, Mao S, Zhan Z, Yu K, He C, Wang C. Effect of hyperlipidemia on Foxp3 expression in apolipoprotein E-knockout mice. J Cardiovasc Med (Hagerstown). 2014; 15:273–279.37. Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006; 12:178–180.38. Gotsman I, Grabie N, Gupta R, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006; 114:2047–2055.39. Mallat Z, Gojova A, Marchiol-Fournigault C, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001; 89:930–934.40. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441:235–238.41. Lin J, Li M, Wang Z, He S, Ma X, Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res. 2010; 51:1208–1217.42. Maganto-García E, Bu DX, Tarrio ML, et al. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011; 187:3521–3529.43. Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008; 103:1220–1231.44. Smeets E, Meiler S, Lutgens E. Lymphocytic tumor necrosis factor receptor superfamily co-stimulatory molecules in the pathogenesis of atherosclerosis. Curr Opin Lipidol. 2013; 24:518–524.45. Michallet MC, Rota G, Maslowski K, Guarda G. Innate receptors for adaptive immunity. Curr Opin Microbiol. 2013; 16:296–302.46. Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013; 34:511–519.47. Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015; 94:193–205.48. Lutgens E, Lievens D, Beckers L, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010; 207:391–404.49. van Wanrooij EJ, van Puijvelde GH, de Vos P, Yagita H, van Berkel TJ, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007; 27:204–210.50. Olofsson PS, Söderström LA, Wågsäter D, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008; 117:1292–1301.51. Jeon HJ, Choi JH, Jung IH, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010; 121:1124–1133.52. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003; 3:609–620.53. Makkouk A, Chester C, Kohrt HE. Rationale for anti-CD137 cancer immunotherapy. Eur J Cancer. 2016; 54:112–119.54. Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014; 47:122–129.55. Kwon BS, Hurtado JC, Lee ZH, et al. Vinay DS. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002; 168:5483–5490.56. Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol. 2005; 174:6803–6808.57. Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004; 173:4218–4229.58. Choi BK, Kim YH, Kwon PM, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009; 182:4107–4115.59. Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012; 189:2697–2701.60. Yan J, Gong J, Liu P, Wnag C, Chen G. Positive correlation between CD137 expression and complex stenosis morphology in patients with acute coronary syndromes. Clin Chim Acta. 2011; 412:993–998.61. Dongming L, Zuxun L, Liangjie X, Biao W, Ping Y. Enhanced levels of soluble and membrane-bound CD137 levels in patients with acute coronary syndromes. Clin Chim Acta. 2010; 411:406–410.62. Yan J, Wang C, Wang Z, Yuan W. The effect of CD137-CD137 ligand interaction on phospholipase C signaling pathway in human endothelial cells. Chem Biol Interact. 2013; 206:256–261.63. Yu Y, He Y, Yang TT, et al. Elevated plasma levels and monocyte-associated expression of CD137 ligand in patients with acute atherothrombotic stroke. Eur Rev Med Pharmacol Sci. 2014; 18:1525–1532.64. Yan J, Wang C, Chen R, Yang H. Clinical implications of elevated serum soluble CD137 levels in patients with acute coronary syndrome. Clinics (Sao Paulo). 2013; 68:193–198.65. Li Y, Yan J, Wu C, Wang Z, Yuan W, Wang D. CD137-CD137L interaction regulates atherosclerosis via cyclophilin A in apolipoprotein E-deficient mice. PLoS One. 2014; 9:e88563.66. Silvestre-Roig C, de Winther M, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014; 114:214–226.67. Choi ET, Collins ET, Marine LA, et al. Matrix metalloproteinase-9 modulation by resident arterial cells is responsible for injury-induced accelerated atherosclerotic plaque development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005; 25:1020–1025.68. Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006; 26:1120–1125.69. Mittal B, Mishra A, Srivastava A, Kumar S, Garg N. Matrix metalloproteinases in coronary artery disease. Adv Clin Chem. 2014; 64:1–72.70. Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006; 116:59–69.71. Chen Y, Aratani Y, Osawa T, Fukuyama N, Tsuji C, Nakazawa H. Activation of inducible nitric oxide synthase increases MMP-2 and MMP-9 levels in ApoE-knockout mice. Tokai J Exp Clin Med. 2008; 33:28–34.72. Wei DH, Jia XY, Liu YH, et al. Cathepsin L stimulates autophagy and inhibits apoptosis of ox-LDL-induced endothelial cells: potential role in atherosclerosis. Int J Mol Med. 2013; 31:400–406.73. Kitamoto S, Sukhova GK, Sun J, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007; 115:2065–2075.74. Sukhova GK, Zhang Y, Pan JH, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003; 111:897–906.75. Guo J, Bot I, de Nooijer R, et al. Leucocyte cathepsin K affects atherosclerotic lesion composition and bone mineral density in low-density lipoprotein receptor deficient mice. Cardiovasc Res. 2009; 81:278–285.76. Jaffer FA, Kim DE, Quinti L, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007; 115:2292–2298.77. Samokhin AO, Wong A, Saftig P, Brömme D. Role of cathepsin K in structural changes in brachiocephalic artery during progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008; 200:58–68.78. Levick SP, Goldspink PH. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev. 2014; 19:227–236.79. Harvey EJ, Ramji DP. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res. 2005; 67:11–20.80. Smith MA, Moylan JS, Smith JD, Li W, Reid MB. IFN-gamma does not mimic the catabolic effects of TNF-alpha. Am J Physiol Cell Physiol. 2007; 293:C1947–C1952.81. Scott RA, Panitch A. Decorin mimic regulates platelet-derived growth factor and interferon-γ stimulation of vascular smooth muscle cells. Biomacromolecules. 2014; 15:2090–2103.82. Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006; 69:625–635.83. Yan J, Chen G, Gong J, Wang C, Du R. Upregulation of OX40-OX40 ligand system on T lymphocytes in patients with acute coronary syndromes. J Cardiovasc Pharmacol. 2009; 54:451–455.84. Liu DM, Yan JC, Wang CP, et al. The clinical implications of increased OX40 ligand expression in patients with acute coronary syndrome. Clin Chim Acta. 2008; 397:22–26.85. Lee WH, Kim SH, Lee Y, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001; 21:2004–2010.86. Kim SH, Lee WH, Kwon BS, Oh GT, Choi YH, Park JE. Tumor necrosis factor receptor superfamily 12 may destabilize atherosclerotic plaques by inducing matrix metalloproteinases. Jpn Circ J. 2001; 65:136–138.87. Jung IH, Choi JH, Jin J, et al. CD137-inducing factors from T cells and macrophages accelerate the destabilization of atherosclerotic plaques in hyperlipidemic mice. FASEB J. 2014; 28:4779–4791.88. Choi JH, Cheong C, Dandamudi DB, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011; 35:819–831.89. Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol. 2002; 72:35–42.90. Choi BK, Kim YH, Kwon PM, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009; 182:4107–4115.91. Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Chen H. Effects of 4-1BB signaling on the biological function of murine dendritic cells. Oncol Lett. 2012; 3:477–481.92. Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012; 189:2697–2701.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is CD137 Ligand (CD137L) Signaling a Fine Tuner of Immune Responses?
- Regulation of Inflammation by Bidirectional Signaling through CD137 and Its Ligand
- CD137-CD137 Ligand Interactions in Inflammation
- Contrasting Roles of Different Endoglin Forms in Atherosclerosis
- Mechanisms of the Wnt Pathways as a Potential Target Pathway in Atherosclerosis