Immune Netw.
2016 Oct;16(5):296-304. 10.4110/in.2016.16.5.296.
Cytokine-like Activity of Liver Type Fatty Acid Binding Protein (L-FABP) Inducing Inflammatory Cytokine Interleukin-6
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Jeju National University School of Medicine, Jeju 63243, Korea.
- 2Department of Medicine, University of Colorado Denver, Aurora, Colorado 80045, USA.
- 3Laboratory of Cytokine Immunology, Department of Biomedical Science and Technology, Konkuk University, Seoul 05029, Korea. soohyun@konkuk.ac.kr
- 4YbdYbiotech research center, Seoul 08589, Korea.
- 5College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 6Department of Medicine, Pusan Paik Hospital, College of Medicine, Inje University, Busan 47392, Korea.
- KMID: 2355023
- DOI: http://doi.org/10.4110/in.2016.16.5.296
Abstract
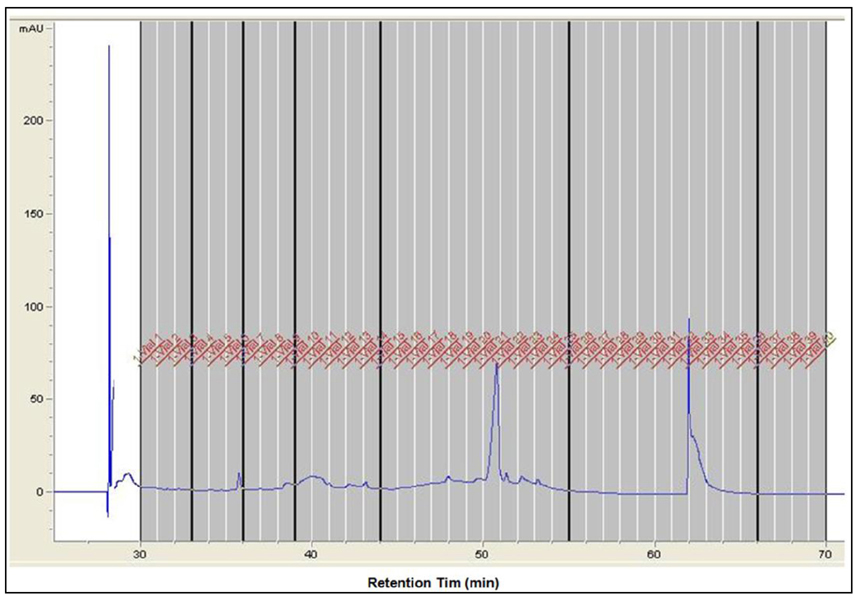
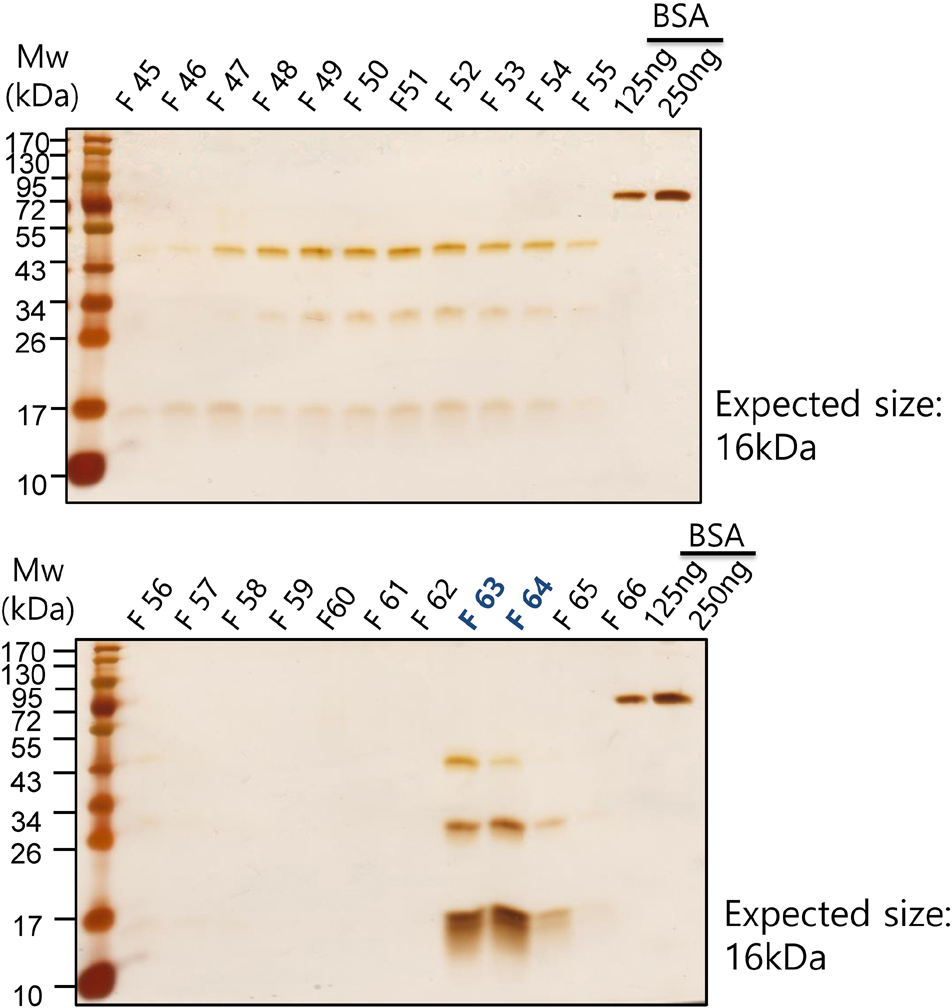
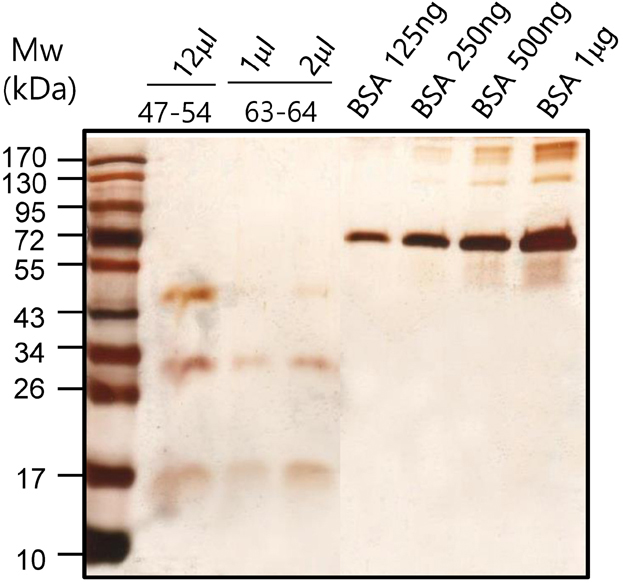
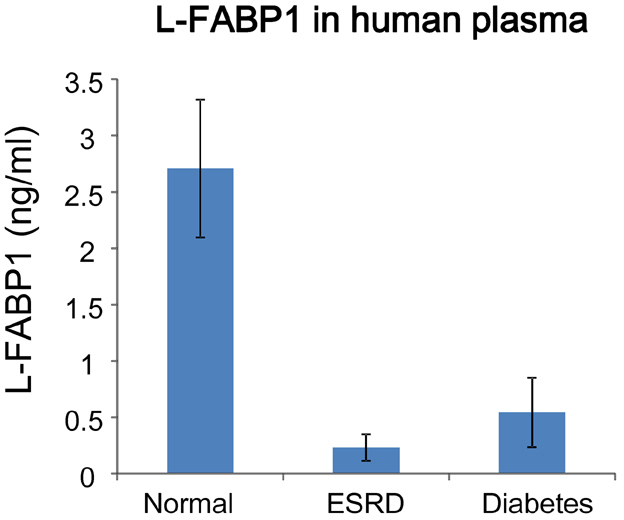
- It has been reported that fatty acid binding proteins (FABPs) do not act only as intracellular mediators of lipid responses but also have extracellular functions. This study aimed to investigate whether extracellular liver type (L)-FABP has a biological activity and to determined serum L-FABP levels in patients with end-stage renal disease (ESRD). We isolated L-FABP complementary deoxyribonucleic acid (cDNA) from the Huh7 human hepatocarcinoma cell line and expressed the recombinant L-FABP protein in Escherichia coli. A549 lung carcinoma and THP-1 monocytic cells were stimulated with the human recombinant L-FABP. Human whole blood cells were also treated with the human recombinant L-FABP or interleukin (IL)-1α. IL-6 levels were measured in cell culture supernatants using IL-6 enzyme-linked immunosorbent assay (ELISA). Human recombinant L-FABP induced IL-6 in a dose-dependent manner in A549, THP-1 cells, and whole blood cells. The blood samples of healthy volunteers and patients with ESRD were taken after an overnight fast. The serum levels of L-FABP in healthy volunteers and ESRD patients were quantified with L-FABP ELISA. The values of L-FABP in patients with ESRD were significantly lower than those in the control group. Our results demonstrated the biological activity of L-FABP in human cells suggesting L-FABP can be a mediator of inflammation.
MeSH Terms
-
Blood Cells
Carrier Proteins*
Cell Culture Techniques
Cell Line
DNA
Enzyme-Linked Immunosorbent Assay
Escherichia coli
Fatty Acid-Binding Proteins
Healthy Volunteers
Humans
Inflammation
Interleukin-6*
Interleukins
Kidney Failure, Chronic
Liver*
Lung
Carrier Proteins
DNA
Fatty Acid-Binding Proteins
Interleukin-6
Interleukins
Figure
Cited by 2 articles
-
Species Specific Antiviral Activity of Porcine Interferon-α8 (IFNα8)
Eunhye Kim, Hyunjhung Jhun, Joohee Kim, Unjoo Park, Seunghyun Jo, Areum Kwak, Sinae Kim, Tam T. Nguyen, Yongsun Kang, Insoo Choi, Joongbok Lee, Heijun Kim, Younghyun Kim, Siyoung Lee, Soohyun Kim
Immune Netw. 2017;17(6):424-436. doi: 10.4110/in.2017.17.6.424.L1 Recombinant Proteins of HPV Tested for Antibody Forming Using Sera of HPV Quadrivalent Vaccine
Begum Akuzum, Sinae Kim, Tam Thanh Nguyen, Jeawoo Hong, Siyoung Lee, Eunhye Kim, Joohee Kim, Yeook Choi, Hyunjhung Jhun, Youngmin Lee, Hyunwoo Kim, Dong Hyun Sohn, Soohyun Kim
Immune Netw. 2018;18(3):. doi: 10.4110/in.2018.18.e19.
Reference
-
1. Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008; 7:489–503.
Article2. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010; 21:1015–1032.
Article3. Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996; 110:339–343.
Article4. Piumngam P, Schachtrup C, Owada Y, Kondo H, Promptmas C, Spener F. Expression of liver-type fatty acid-binding protein in murine lung and its release into serum upon challenge of lung with lipopolysaccharide. Eur J Lipid Sci Technol. 2005; 107:145–152.
Article5. Pelsers MM, Morovat A, Alexander GJ, Hermens WT, Trull AK, Glatz JF. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002; 48:2055–2057.
Article6. Monbaliu D, de VB, Crabbe T, van HE, Verwaest C, Roskams T, Fevery J, Pirenne J, Buurman WA. Liver fatty acid-binding protein: an early and sensitive plasma marker of hepatocellular damage and a reliable predictor of graft viability after liver transplantation from non-heart-beating donors. Transplant Proc. 2005; 37:413–416.
Article7. Akbal E, Koklu S, Kocak E, Cakal B, Gunes F, Basar O, Tuna Y, Senes M. Liver fatty acid-binding protein is a diagnostic marker to detect liver injury due to chronic hepatitis C infection. Arch Med Res. 2013; 44:34–38.
Article8. Morrissey PE, Gollin G, Marks WH. Small bowel allograft rejection detected by serum intestinal fatty acid-binding protein is reversible. Transplantation. 1996; 61:1451–1455.
Article9. Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003; 36:529–535.
Article10. Okamoto F, Sohmiya K, Ohkaru Y, Kawamura K, Asayama K, Kimura H, Nishimura S, Ishii H, Sunahara N, Tanaka T. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin Chem Lab Med. 2000; 38:231–238.
Article11. Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am J Med. 2002; 113:717–722.
Article12. Pelsers MM, Hanhoff T, d Van V, Arts B, Peters M, Ponds R, Honig A, Rudzinski W, Spener F, de K Jr, Twijnstra A, Hermens WT, Menheere PP, Glatz JF. Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin Chem. 2004; 50:1568–1575.
Article13. Foucaud L, Grillasca J, Niot I, Domingo N, Lafont H, Planells R, Besnard P. Output of liver fatty acidbinding protein (L-FABP) in bile. Biochim Biophys Acta. 1999; 1436:593–599.
Article14. Specht B, Bartetzko N, Hohoff C, Kuhl H, Franke R, Borchers T, Spener F. Mammary derived growth inhibitor is not a distinct protein but a mix of heart-type and adipocyte-type fatty acid-binding protein. J Biol Chem. 1996; 271:19943–19949.
Article15. Bronsky J, Karpisek M, Bronska E, Pechova M, Jancikova B, Kotolova H, Stejskal D, Prusa R, Nevoral J. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: proteins newly identified in human breast milk. Clin Chem. 2006; 52:1763–1770.
Article16. Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006; 52:405–413.
Article17. Mita T, Furuhashi M, Hiramitsu S, Ishii J, Hoshina K, Ishimura S, Fuseya T, Watanabe Y, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring). 2015; 23:359–367.
Article18. Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart-Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009; 105:326–334.
Article19. Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, Tuncman G, Hotamisligil GS. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013; 17:768–778.
Article20. Kralisch S, Kloting N, Ebert T, Kern M, Hoffmann A, Krause K, Jessnitzer B, Lossner U, Sommerer I, Stumvoll M, Fasshauer M. Circulating adipocyte fatty acid-binding protein induces insulin resistance in mice in vivo. Obesity (Silver Spring). 2015; 23:1007–1013.
Article21. Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Omata M, Kimura K. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006; 284:175–182.
Article22. Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007; 18:2894–2902.
Article23. Matsui K, Kamijo-Ikemori A, Imai N, Sugaya T, Yasuda T, Tatsunami S, Toyama T, Shimizu M, Furuichi K, Wada T, Shibagaki Y, Kimura K. Clinical significance of urinary liver-type fatty acid-binding protein as a predictor of ESRD and CVD in patients with CKD. Clin Exp Nephrol. 2016; 20:195–203.
Article24. Lachmann RA, Werchan S, Schachtrup C, Haitsma JJ, Spener F, Lachmann B. Liver-type fatty acid binding protein in serum and broncho-alveolar lavage in a model of acute respiratory failure because of surfactant depletion--a possible marker for lung damage? Clin Physiol Funct Imaging. 2006; 26:371–375.
Article25. Kawai A, Kusaka M, Kitagawa F, Ishii J, Fukami N, Maruyama T, Sasaki H, Shiroki R, Kurahashi H, Hoshinaga K. Serum liver-type fatty acid-binding protein predicts recovery of graft function after kidney transplantation from donors after cardiac death. Clin Transplant. 2014; 28:749–754.
Article26. Akbal E, Kocak E, Akyurek O, Koklu S, Batgi H, Senes M. Liver fatty acid-binding protein as a diagnostic marker for non-alcoholic fatty liver disease. Wien Klin Wochenschr. 2016; 128:48–52.
Article27. Shi J, Zhang Y, Gu W, Cui B, Xu M, Yan Q, Wang W, Ning G, Hong J. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PLoS One. 2012; 7:e48777.
Article28. Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Miura T. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 2013; 8:e81318.
Article29. Kwan BC, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007; 18:1246–1261.
Article30. Jin K, Norris K, Vaziri ND. Dysregulation of hepatic fatty acid metabolism in chronic kidney disease. Nephrol Dial Transplant. 2013; 28:313–320.
Article31. Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol. 2014; 13:126.
Article32. Yeung DC, Xu A, Tso AW, Chow WS, Wat NM, Fong CH, Tam S, Sham PC, Lam KS. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009; 32:132–134.
Article33. Girona J, Rosales R, Plana N, Saavedra P, Masana L. FABP4 induces vascular smooth muscle cell proliferation and migration through a MAPK-dependent pathway. PLoS One. 2013; 8:e81914.
Article34. Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM, Fong DY, Fong CH, Chau MT, Sham PC, Lam KS. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J. 2008; 29:2156–2163.
Article35. Bagheri R, Qasim AN, Mehta NN, Terembula K, Kapoor S, Braunstein S, Schutta M, Iqbal N, Lehrke M, Reilly MP. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol. 2010; 106:1118–1123.
Article36. Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014; 24:31–44.
Article37. Brunialti MK, Kallas EG, Freudenberg M, Galanos C, Salomao R. Influence of EDTA and heparin on lipopolysaccharide binding and cell activation, evaluated at single-cell level in whole blood. Cytometry. 2002; 50:14–18.
Article38. Duvigneau JC, Sipos W, Hartl RT, Bayer M, Moldzio R, Stevenson L, Adair B, Gemeiner M. Heparin and EDTA as anticoagulant differentially affect cytokine mRNA level of cultured porcine blood cells. J Immunol Methods. 2007; 324:38–47.
Article39. Freitas M, Porto G, Lima JL, Fernandes E. Isolation and activation of human neutrophils in vitro The importance of the anticoagulant used during blood collection. Clin Biochem. 2008; 41:570–575.
Article40. Iwamoto M, Miyoshi T, Doi M, Takeda K, Kajiya M, Nosaka K, Nakayama R, Hirohata S, Usui S, Kusachi S, Sakane K, Nakamura K, Ito H. Elevated serum adipocyte fatty acid-binding protein concentrations are independently associated with renal dysfunction in patients with stable angina pectoris. Cardiovasc Diabetol. 2012; 11:26.
Article41. Furuhashi M, Ura N, Hasegawa K, Yoshida H, Tsuchihashi K, Nakata T, Shimamoto K. Serum ratio of heart-type fatty acid-binding protein to myoglobin A novel marker of cardiac damage and volume overload in hemodialysis patients. Nephron Clin Pract. 2003; 93:C69–C74.42. Furuhashi M, Ura N, Hasegawa K, Tsuchihashi K, Nakata T, Shimamoto K. Utility of serum ratio of heart-type fatty acid-binding protein to myoglobin for cardiac damage regardless of renal dysfunction. Circ J. 2004; 68:656–659.
Article43. Sommer G, Ziegelmeier M, Bachmann A, Kralisch S, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD). Clin Endocrinol (Oxf). 2008; 69:901–905.
Article44. Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, Shimamoto K, Hotamisligil GS, Miura T. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 2011; 6:e27356.
Article45. Mori Y, Hirano T, Nagashima M, Shiraishi Y, Fukui T, Adachi M. Decreased peroxisome proliferator-activated receptor alpha gene expression is associated with dyslipidemia in a rat model of chronic renal failure. Metabolism. 2007; 56:1714–1718.
Article46. Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998; 98:2088–2093.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The associations of Urinary Neutrophil Gelatinaseassociated Lipocalin (NGAL) and Liver-type Fatty Acidbinding Protein (L-FABP) Levels with Hematuria in Children and Adolescents
- Association of Serum Adipocyte-Specific Fatty Acid Binding Protein with Fatty Liver Index as a Predictive Indicator of Nonalcoholic Fatty Liver Disease
- The Role of Urinary Liver-Type Fatty Acid-Binding Protein in Critically Ill Patients
- Regulation of mFABP (fatty acid binding protein) Expression by PPAR in Cultured Human Skeletal Muscle Cell
- Tissue Specific Expression of Lipid Metabolism Related Molecules in Digestive Organs of Miniature Pigs