Immune Netw.
2016 Oct;16(5):286-295. 10.4110/in.2016.16.5.286.
Enhanced Viral Replication by Cellular Replicative Senescence
- Affiliations
-
- 1Brain Korea 21 Plus for Biomedical Science, College of Medicine, Korea University, Seoul 08308, Korea. oshin@korea.ac.kr
- KMID: 2355022
- DOI: http://doi.org/10.4110/in.2016.16.5.286
Abstract
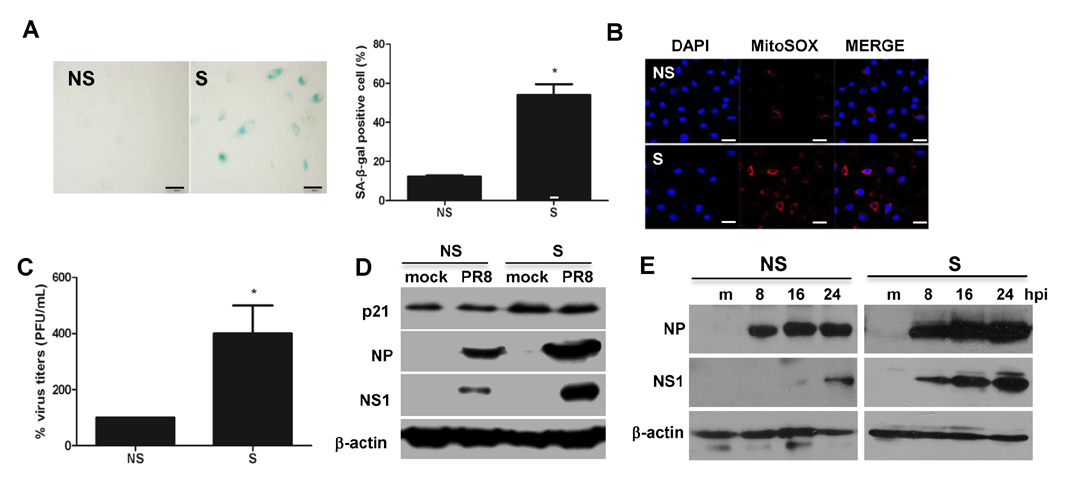
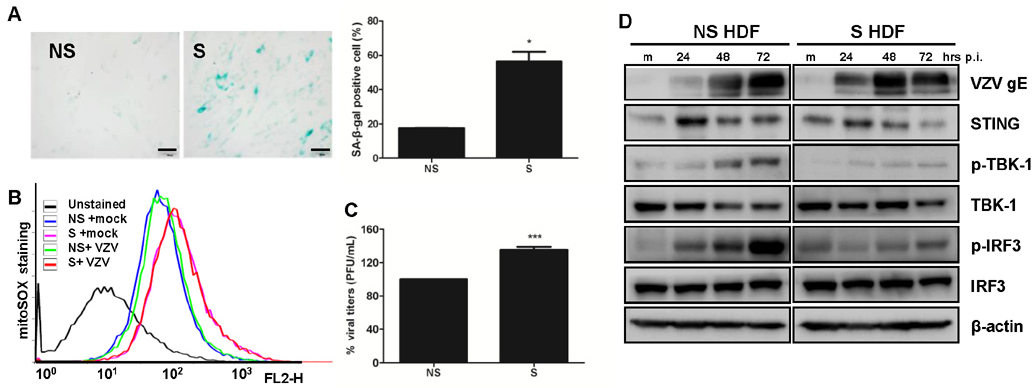
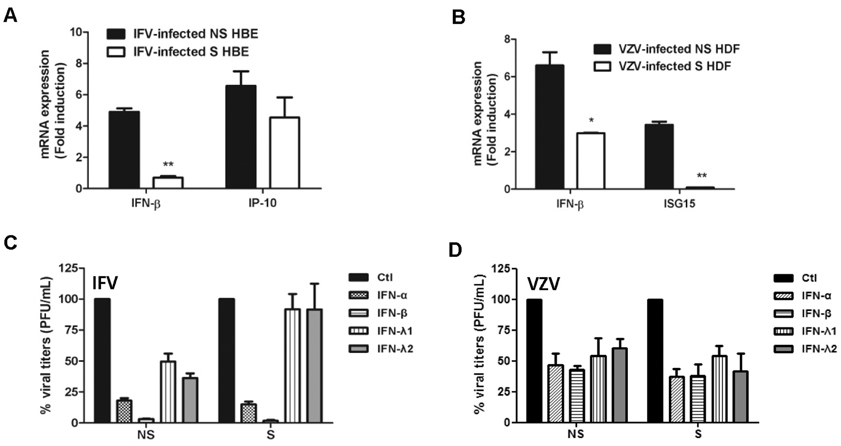
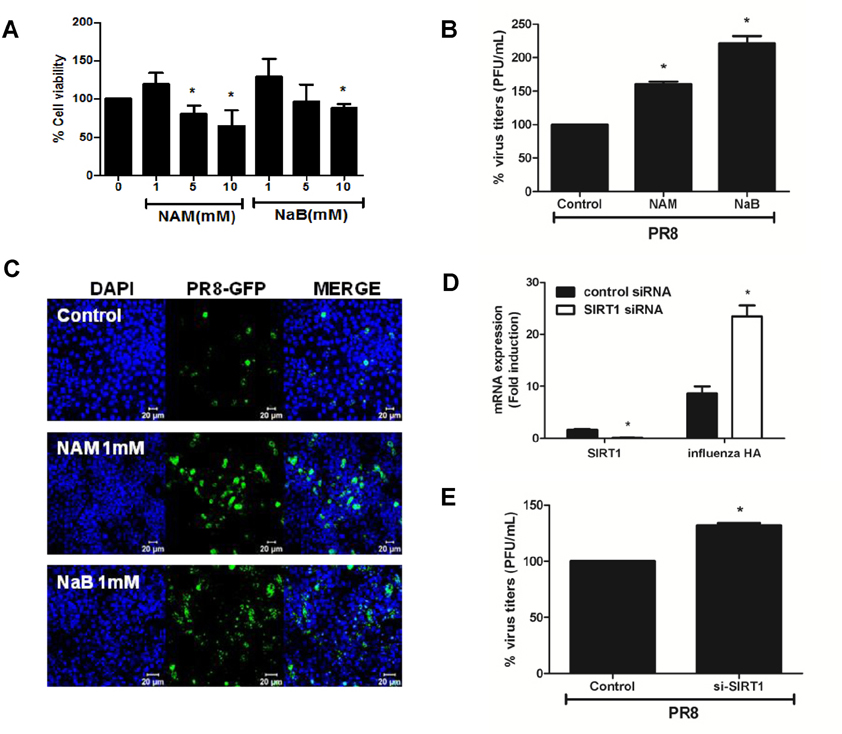
- Cellular replicative senescence is a major contributing factor to aging and to the development and progression of aging-associated diseases. In this study, we sought to determine viral replication efficiency of influenza virus (IFV) and Varicella Zoster Virus (VZV) infection in senescent cells. Primary human bronchial epithelial cells (HBE) or human dermal fibroblasts (HDF) were allowed to undergo numbers of passages to induce replicative senescence. Induction of replicative senescence in cells was validated by positive senescence-associated β-galactosidase staining. Increased susceptibility to both IFV and VZV infection was observed in senescent HBE and HDF cells, respectively, resulting in higher numbers of plaque formation, along with the upregulation of major viral antigen expression than that in the non-senescent cells. Interestingly, mRNA fold induction level of virus-induced type I interferon (IFN) was attenuated by senescence, whereas IFN-mediated antiviral effect remained robust and potent in virus-infected senescent cells. Additionally, we show that a longevity-promoting gene, sirtuin 1 (SIRT1), has antiviral role against influenza virus infection. In conclusion, our data indicate that enhanced viral replication by cellular senescence could be due to senescence-mediated reduction of virus-induced type I IFN expression.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Aging and the Immune System: the Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity
Soo-Jin Oh, Jae Kyung Lee, Ok Sarah Shin
Immune Netw. 2019;19(6):. doi: 10.4110/in.2019.19.e37.
Reference
-
1. van Deursen JM. The role of senescent cells in ageing. Nature. 2014; 509:439–446.
Article2. Hwang ES. Replicative senescence and senescence-like state induced in cancer-derived cells. Mech Ageing Dev. 2002; 123:1681–1694.
Article3. Campisi J. The biology of replicative senescence. Eur J Cancer. 1997; 33:703–709.
Article4. Dorrington MG, Bowdish DM. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol. 2013; 4:171.
Article5. Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis. 2012; 3:68–90.6. Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005; 352:2266–2267.
Article7. Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008; 197:825–835.
Article8. Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010; 184:2518–2527.
Article9. Wong CP, Magnusson KR, Ho E. Aging is associated with altered dendritic cells subset distribution and impaired proinflammatory cytokine production. Exp Gerontol. 2010; 45:163–169.
Article10. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013; 13:875–887.
Article11. Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010; 22:507–513.
Article12. Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011; 203:1415–1424.
Article13. Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age:insights into inflammaging. Longev. Healthspan. 2013; 2:8.14. Haigis MC, Sinclair DA. Mammalian sirtuins:biological insights and disease relevance. Annu Rev Pathol. 2010; 5:253–295.15. Chen H, Wan Y, Zhou S, Lu Y, Zhang Z, Zhang R, Chen F, Hao D, Zhao X, Guo Z, Liu D, Liang C. Endothelium-specific SIRT1 overexpression inhibits hyperglycemia-induced upregulation of vascular cell senescence. Sci China Life Sci. 2012; 55:467–473.
Article16. Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, Shenk T, Cristea IM. Sirtuins are evolutionarily conserved viral restriction factors. MBio. 2014; 5.
Article17. Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A. 2010; 107:11531–11536.
Article18. Jeon JS, Won YH, Kim IK, Ahn JH, Shin OS, Kim JH, Lee CH. Analysis of single nucleotide polymorphism among varicella-zoster virus and identification of vaccine-specific sites. Virology. 2016; 496:277–286.
Article19. Choi EJ, Lee CH, Kim YC, Shin OS. Wogonin inhibits Varicella-Zoster (shingles) virus replication via modulation of type I interferon signaling and adenosine monophosphate-activated protein kinase activity. J Funct Foods. 2015; 17:399–409.
Article20. Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000; 113(Pt 20):3613–3622.
Article21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article22. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961; 25:585–621.
Article23. Moffat J, Ito H, Sommer M, Taylor S, Arvin AM. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J Virol. 2002; 76:8468–8471.
Article24. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes:a complex web of host defenses. Annu Rev Immunol. 2014; 32:513–545.25. Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005; 37:961–976.
Article26. Weiland T, Lampe J, Essmann F, Venturelli S, Berger A, Bossow S, Berchtold S, Schulze-Osthoff K, Lauer UM, Bitzer M. Enhanced killing of therapy-induced senescent tumor cells by oncolytic measles vaccine viruses. Int J Cancer. 2014; 134:235–243.
Article27. Kim JA, Park SK, Kumar M, Lee CH, Shin OS. Insights into the role of immunosenescence during varicella zoster virus infection (shingles) in the aging cell model. Oncotarget. 2015; 6:35324–35343.
Article28. Targonski PV, Jacobson RM, Poland GA. Immunosenescence:role and measurement in influenza vaccine response among the elderly. Vaccine. 2007; 25:3066–3069.
Article29. Mitzel DN, Jacobson RM, Poland GA. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN-beta production during Streptococcus pneumoniae infection. J Immunol. 2014; 192:4273–4283.
Article30. Kida Y, Goligorsky MS. Sirtuins, cell senescence, and vascular aging. Can J Cardiol. 2016; 32:634–641.
Article31. Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol Lett. 2013; 6:600–604.
Article32. Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003; 42:9249–9256.
Article33. He M, Gao SJ. A novel role of SIRT1 in gammaherpesvirus latency and replication. Cell Cycle. 2014; 13:3328–3330.
Article34. Pagans SA. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005; 3:e41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Kidneys with bad ends
- The Role of Cellular Senescence in the Gastrointestinal Mucosa
- Possible Roles of UL112-113 Proteins in Human Cytomegalovirus DNA Replication
- Effects of Replicative Senescence on the Cell Cycle Regulation in Human Gingival Fibroblasts