Diabetes Metab J.
2016 Oct;40(5):406-413. 10.4093/dmj.2016.40.5.406.
Rg3 Improves Mitochondrial Function and the Expression of Key Genes Involved in Mitochondrial Biogenesis in C2C12 Myotubes
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. kspark@snu.ac.kr
- 2Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea.
- 3Department of Biomedical Sciences, Seoul National University Graduate School, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 5Biomedical Research Institute and Innovative Research Institute for Cell Therapy, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2354614
- DOI: http://doi.org/10.4093/dmj.2016.40.5.406
Abstract
- BACKGROUND
Panax ginseng has glucose-lowering effects, some of which are associated with the improvement in insulin resistance in skeletal muscle. Because mitochondria play a pivotal role in the insulin resistance of skeletal muscle, we investigated the effects of the ginsenoside Rg3, one of the active components of P. ginseng, on mitochondrial function and biogenesis in C2C12 myotubes.
METHODS
C2C12 myotubes were treated with Rg3 for 24 hours. Insulin signaling pathway proteins were examined by Western blot. Cellular adenosine triphosphate (ATP) levels and the oxygen consumption rate were measured. The protein or mRNA levels of mitochondrial complexes were evaluated by Western blot and quantitative reverse transcription polymerase chain reaction analysis.
RESULTS
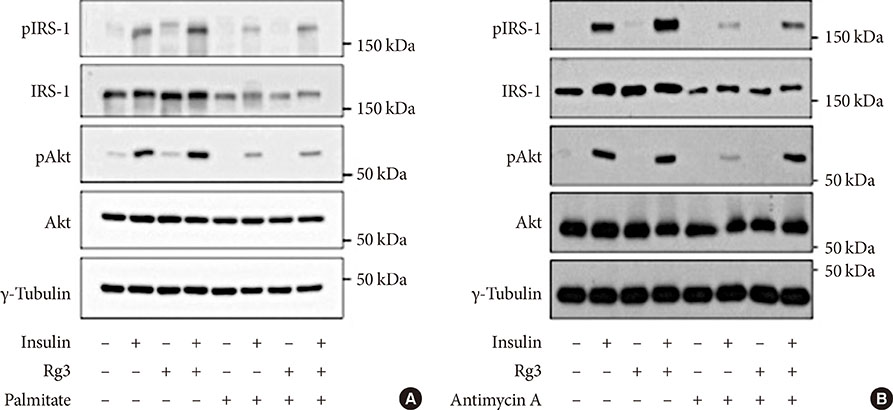
Rg3 treatment to C2C12 cells activated the insulin signaling pathway proteins, insulin receptor substrate-1 and Akt. Rg3 increased ATP production and the oxygen consumption rate, suggesting improved mitochondrial function. Rg3 increased the expression of peroxisome proliferator-activated receptor γ coactivator 1α, nuclear respiratory factor 1, and mitochondrial transcription factor, which are transcription factors related to mitochondrial biogenesis. Subsequent increased expression of mitochondrial complex IV and V was also observed.
CONCLUSION
Our results suggest that Rg3 improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis, leading to an improvement in insulin resistance in skeletal muscle. Rg3 may have the potential to be developed as an anti-hyperglycemic agent.
Keyword
MeSH Terms
-
Adenosine Triphosphate
Blotting, Western
Insulin
Insulin Receptor Substrate Proteins
Insulin Resistance
Mitochondria
Muscle Fibers, Skeletal*
Muscle, Skeletal
Nuclear Respiratory Factor 1
Organelle Biogenesis*
Oxygen Consumption
Panax
Peroxisomes
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Transcription Factors
Adenosine Triphosphate
Insulin
Insulin Receptor Substrate Proteins
Nuclear Respiratory Factor 1
RNA, Messenger
Transcription Factors
Figure
Reference
-
1. Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000; 160:1009–1013.2. Vuksan V, Stavro MP, Sievenpiper JL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Xu Z. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000; 23:1221–1226.3. De Souza LR, Jenkins AL, Sievenpiper JL, Jovanovski E, Rahelic D, Vuksan V. Korean red ginseng (Panax ginseng C.A. Meyer) root fractions: differential effects on postprandial glycemia in healthy individuals. J Ethnopharmacol. 2011; 137:245–250.4. Yoon JW, Kang SM, Vassy JL, Shin H, Lee YH, Ahn HY, Choi SH, Park KS, Jang HC, Lim S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig. 2012; 3:309–317.5. Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002; 51:1851–1858.6. Lim S, Yoon JW, Choi SH, Cho BJ, Kim JT, Chang HS, Park HS, Park KS, Lee HK, Kim YB, Jang HC. Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin-resistant rat model. Metabolism. 2009; 58:8–15.7. Yun SN, Ko SK, Lee KH, Chung SH. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch Pharm Res. 2007; 30:587–595.8. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693.9. Kim M, Ahn BY, Lee JS, Chung SS, Lim S, Park SG, Jung HS, Lee HK, Park KS. The ginsenoside Rg3 has a stimulatory effect on insulin signaling in L6 myotubes. Biochem Biophys Res Commun. 2009; 389:70–73.10. Kelly SP, Wood CM. Prolactin effects on cultured pavement cell epithelia and pavement cell plus mitochondria-rich cell epithelia from freshwater rainbow trout gills. Gen Comp Endocrinol. 2002; 128:44–56.11. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005; 54:8–14.12. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005; 1:15–25.13. Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004; 36:28–34.14. Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. . Physiology (Bethesda). 2006; 21:48–60.15. Park MW, Ha J, Chung SH. 20(S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol Pharm Bull. 2008; 31:748–751.16. DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985; 76:149–155.17. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414:799–806.18. Tian J, Zhang S, Li G, Liu Z, Xu B. 20(S)-ginsenoside Rg3, a neuroprotective agent, inhibits mitochondrial permeability transition pores in rat brain. Phytother Res. 2009; 23:486–491.19. Sun M, Huang C, Wang C, Zheng J, Zhang P, Xu Y, Chen H, Shen W. Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. Biochem Biophys Res Commun. 2013; 441:169–174.20. Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009; 71:177–203.21. Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007; 355:734–739.22. Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J Biol Chem. 2010; 285:142–152.23. O'Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. 2013; 37:1–21.24. Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005; 19:1146–1148.25. Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007; 104:12017–12022.26. Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. . J Appl Physiol (1985). 2008; 104:429–438.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Mitochondrial Biogenesis Dysfunction in Diabetic Cardiomyopathy
- Ursolic acid improves the indoxyl sulfate-induced impairment of mitochondrial biogenesis in C2C12 cells
- Effects of isorhamnetin on the regulation of mitochondrial function in C2C12 muscle cells
- Cellular NAD⺠Level: A Key Determinant of Mitochondrial Quality and Health
- Effects of oxypeucedanin hydrate isolated from Angelica dahurica on myoblast differentiation in association with mitochondrial function