Ann Dermatol.
2015 Jun;27(3):257-264. 10.5021/ad.2015.27.3.257.
HR-1 Mice: A New Inflammatory Acne Mouse Model
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. weonju@knu.ac.kr
- KMID: 2352495
- DOI: http://doi.org/10.5021/ad.2015.27.3.257
Abstract
- BACKGROUND
There is no appropriate in vivo animal model that reflects the inflammatory response of human acne.
OBJECTIVE
This study investigated the effect of Propionibacterium acnes on the development of inflammatory acne-like lesions in four mouse strains with different degrees of immune response for the development of an optimal mouse model of inflammatory acne.
METHODS
Human P. acnes suspensions (10(8) and 10(9) colony forming unit [CFU]/microl) were injected into the backs of HR-1, BALB/c, vitamin D receptor-knockout (VDR k/o), and severe combined immunodeficiency disease mice. Inflammation levels were evaluated two weeks after injection of P. acnes suspensions. In addition, histopathological examination and immunohistochemical staining of the expressions of inflammatory biomarkers (i.e., CD4+/CD8+ T lymphocytes, neutrophils, myeloperoxidase, interleukin-1beta, matrix metalloprotease (MMP)-2, MMP-3, MMP-9, toll-like receptor (TLR)-2, LL-37, and integrin alpha6) were performed on tissue specimens.
RESULTS
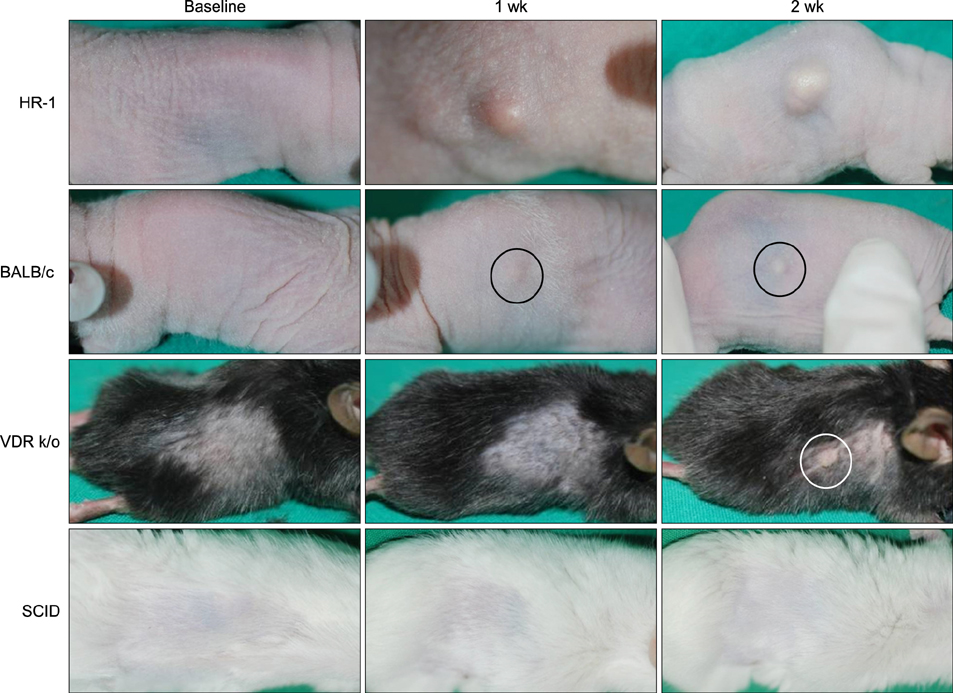
The HR-1 mouse strain exhibited the most remarkable inflammatory reaction with epithelial proliferation and microcomedone-like cyst formation. HR-1 mice also demonstrated aberrant integrin expression in the epidermis around both inflamed lesions and newly formed microcomedones. These findings were more prominent in the group receiving 10(9) CFU/microl P. acnes than 10(8) CFU/microl. MMP-9 expression in HR-1 mice was also upregulated around the microcomedone-like cysts. Finally, expression levels of TLR-2 and LL-37 were higher in HR-1 and BALB/c mice than the VDR k/o and SCID mice strains.
CONCLUSION
P. acnes induces acneiform inflammation with small microcomedones in HR-1 mice. Therefore, the HR-1 mouse strain represents a good candidate for the development of a new inflammatory acne mouse model.
MeSH Terms
-
Acne Vulgaris*
Animals
Biomarkers
Epidermis
Humans
Inflammation
Interleukin-1beta
Mice*
Mice, SCID
Models, Animal
Neutrophils
Peroxidase
Propionibacterium acnes
Severe Combined Immunodeficiency
Stem Cells
Suspensions
T-Lymphocytes
Toll-Like Receptors
Vitamin D
Interleukin-1beta
Peroxidase
Suspensions
Toll-Like Receptors
Vitamin D
Figure
Cited by 1 articles
-
Efficacy of Bacteriophages in Propionibacterium acnes-Induced Inflammation in Mice
Min Ji Kim, Dong Hyuk Eun, Seok Min Kim, Jungmin Kim, Weon Ju Lee
Ann Dermatol. 2019;31(1):22-28. doi: 10.5021/ad.2019.31.1.22.
Reference
-
1. Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013; 27:1–10.2. De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM. Intradermal injection of Propionibacterium acnes: a model of inflammation relevant to acne. J Invest Dermatol. 1984; 83:394–398.
Article3. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003; 121:20–27.
Article4. Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007; 26:195–202.
Article5. Liu Y, Sundberg JP, Das S, Carpenter D, Cain KT, Michaud EJ, et al. Molecular basis for hair loss in mice carrying a novel nonsense mutation (Hrrh-R) in the hairless gene (Hr). Vet Pathol. 2010; 47:167–176.
Article6. Schaffer BS, Grayson MH, Wortham JM, Kubicek CB, McCleish AT, Prajapati SI, et al. Immune competency of a hairless mouse strain for improved preclinical studies in genetically engineered mice. Mol Cancer Ther. 2010; 9:2354–2364.
Article7. Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980; 27:461–467.
Article8. Mathieu C, Van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, et al. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res. 2001; 16:2057–2065.
Article9. Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykkö I, et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009; 115:91–97.
Article10. Milner JD, Fasth A, Etzioni A. Autoimmunity in severe combined immunodeficiency (SCID): lessons from patients and experimental models. J Clin Immunol. 2008; 28:Suppl 1. S29–S33.
Article11. Webster GF. Inflammation in acne vulgaris. J Am Acad Dermatol. 1995; 33:247–253.
Article12. Cunliffe WJ, Holland DB, Clark SM, Stables GI. Comedogenesis: some new aetiological, clinical and therapeutic strategies. Br J Dermatol. 2000; 142:1084–1091.
Article13. Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981; 41:1269–1270.
Article14. Nakano K, Kiyokane K, Benvenuto-Andrade C, González S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol Physiol. 2007; 20:29–36.
Article15. Nakatsuji T, Shi Y, Zhu W, Huang CP, Chen YR, Lee DY, et al. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008; 8:3406–3415.
Article16. Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980; 40:27–32.17. Bechara FG, Sand M, Skrygan M, Kreuter A, Altmeyer P, Gambichler T. Acne inversa: evaluating antimicrobial peptides and proteins. Ann Dermatol. 2012; 24:393–397.
Article18. Jang YH, Sim JH, Kang HY, Kim YC, Lee ES. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the nongranulomatous rosacea. J Eur Acad Dermatol Venereol. 2011; 25:544–548.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Red or Infrared Light-Emitting Diodes in a Mouse Model of Propionibacterium acnes-Induced Inflammation
- Efficacy of Bacteriophages in Propionibacterium acnes-Induced Inflammation in Mice
- Meconopsis quintuplinervia Regel Improves Cutibacterium acnes-Induced Inflammatory Responses in a Mouse Ear Edema Model and Suppresses Pro-Inflammatory Chemokine Production via the MAPK and NF-κB Pathways in RAW264.7 Cells
- Repurposing Auranofin, an Anti-Rheumatic Gold Compound, to Treat Acne Vulgaris by Targeting the NLRP3 Inflammasome
- Shikonin ameliorates salivary gland damage and inflammation in a mouse model of Sjögren’s syndrome by modulating MAPK signaling pathway