Ann Dermatol.
2016 Apr;28(2):186-191. 10.5021/ad.2016.28.2.186.
Efficacy of Red or Infrared Light-Emitting Diodes in a Mouse Model of Propionibacterium acnes-Induced Inflammation
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. weonju@knu.ac.kr
- KMID: 2171375
- DOI: http://doi.org/10.5021/ad.2016.28.2.186
Abstract
- BACKGROUND
Laser/light-based devices may provide an alternative to conventional acne therapeutics in some patients with nonresponsive acne.
OBJECTIVE
We investigated the efficacy of red or infrared light-emitting diode (LED) devices in a mouse model of Propionibacterium acnes-induced inflammation through clinical examination and histopathological and immunohistochemical studies.
METHODS
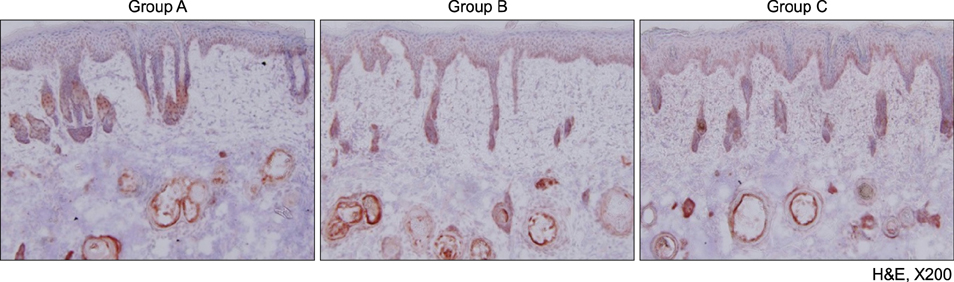
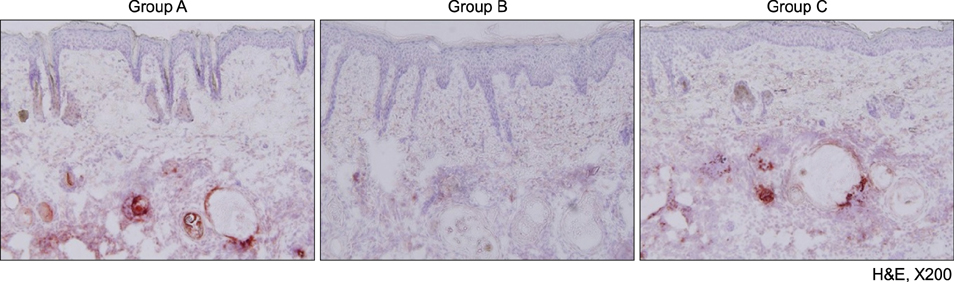
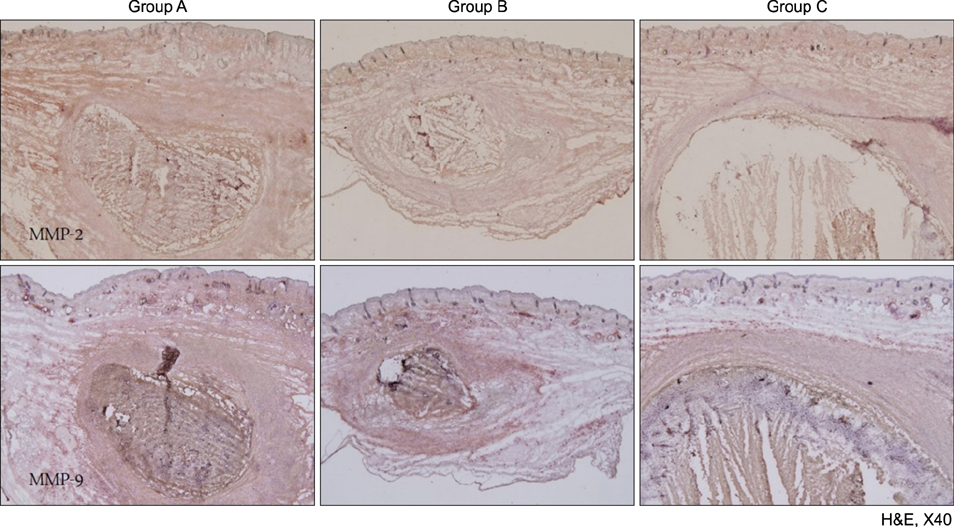
A human-derived Propionibacterium acnes suspension (10(9) colony-forming units /µl) was injected into the back of an HR-1 mouse. Then, a 28.9 J/cm2 650-nm red LED or 9.3 J/cm2 830-nm infrared LED was applied to the mouse with P. acnes-induced inflammation once daily for 2 weeks. Two weeks after treatment, histological findings with hematoxylin and eosin staining and expression levels of inflammatory biomarkers (integrin α6, neutrophils, interleukin [IL]-1β, and matrix metalloproteinase [MMP]-2/9) were evaluated in tissue specimens using immunohistochemical staining.
RESULTS
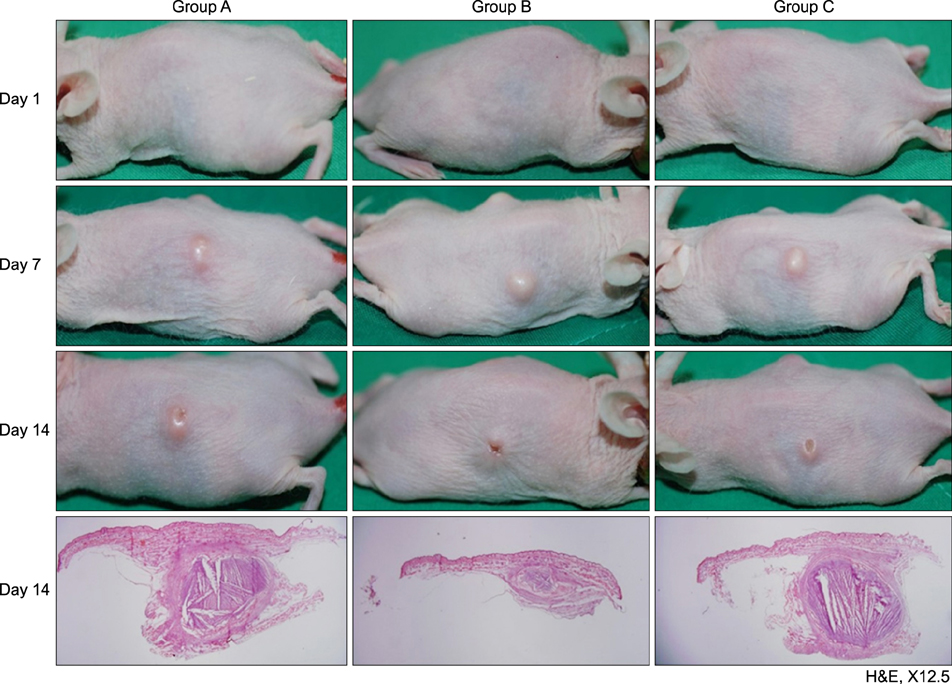
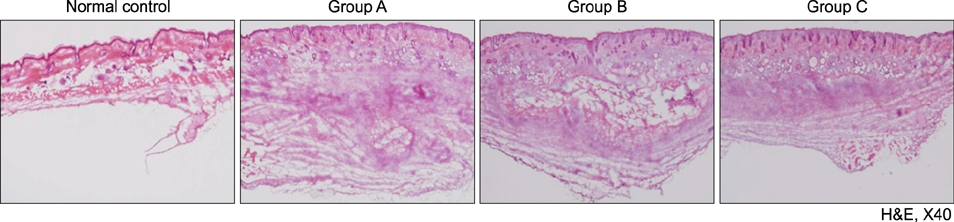
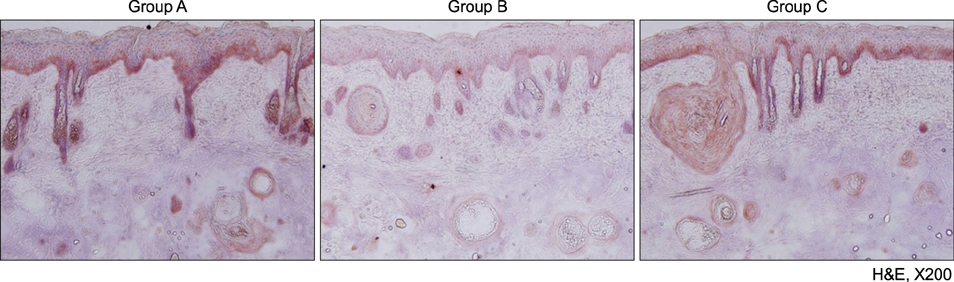
Mice treated with red and infrared LED showed clinical improvement in inflammatory nodules compared to mice in the control group. Red LED was much more effective than infrared LED. Epidermal hyperplasia, comedone-like cysts, and integrin α6 expression improved to a similar extent in the red and infrared LED treatment groups and control group. Neutrophil, IL-1β, MMP-2, and MMP-9 expression after treatment with red and infrared LED decreased considerably compared to expression in the control group.
CONCLUSION
In a mouse model of P. acnes-induced inflammatory nodules, red and infrared LED devices may be an alternative to conventional acne therapies. In addition, a mouse model of P. acnes-induced inflammatory nodules is helpful for laboratory research of acne.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Various Wavelengths of Light-Emitting Diode Light Regulate the Proliferation of Human Dermal Papilla Cells and Hair Follicles via Wnt/β-Catenin and the Extracellular Signal-Regulated Kinase Pathways
Hong Jin Joo, Kwan Ho Jeong, Jung Eun Kim, Hoon Kang
Ann Dermatol. 2017;29(6):747-754. doi: 10.5021/ad.2017.29.6.747.
Reference
-
1. Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013; 27:1–10.2. Huh SY, Na JI, Huh CH, Park KC. The effect of photodynamic therapy using indole-3-acetic Acid and green light on acne vulgaris. Ann Dermatol. 2012; 24:56–60.
Article3. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003; 121:20–27.
Article4. Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007; 26:195–202.
Article5. Mariwalla K, Rohrer TE. Use of lasers and light-based therapies for treatment of acne vulgaris. Lasers Surg Med. 2005; 37:333–342.
Article6. Nakano K, Kiyokane K, Benvenuto-Andrade C, González S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol Physiol. 2007; 20:29–36.
Article7. Nakatsuji T, Shi Y, Zhu W, Huang CP, Chen YR, Lee DY, et al. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008; 8:3406–3415.
Article8. Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC. Phototherapy in the treatment of acne vulgaris: what is its role? Am J Clin Dermatol. 2004; 5:211–216.9. Elman M, Lebzelter J. Light therapy in the treatment of acne vulgaris. Dermatol Surg. 2004; 30:139–146.
Article10. Ross EV. Optical treatments for acne. Dermatol Ther. 2005; 18:253–266.
Article11. Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers Surg Med. 1989; 9:497–505.
Article12. Kjeldstad B. Different photoinactivation mechanisms in Propionibacterium acnes for near-ultraviolet and visible light. Photochem Photobiol. 1987; 46:363–366.
Article13. Goldberg DJ, Russell BA. Combination blue (415 nm) and red (633 nm) LED phototherapy in the treatment of mild to severe acne vulgaris. J Cosmet Laser Ther. 2006; 8:71–75.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of 650 nm Red Light Phototherapy on Acne Vulgaris
- Study of the Photoinactivation Effect on Propionibacterium acnes after Light Irradiation with Variable Wavelengths
- Efficacy of Bacteriophages in Propionibacterium acnes-Induced Inflammation in Mice
- The Effect of Blue Light on Propionibacterium acnes and the Expression of Inflammatory Cytokines in HaCaT Cells
- A study on the chemotactic activity of the peripheral blood neutrop- hils in acne patients to the cytosol antigen of propionibacterium acnes