Transl Clin Pharmacol.
2015 Dec;23(2):49-53. 10.12793/tcp.2015.23.2.49.
Pharmacokinetics and bioequivalence of two different 20 mg olmesartan tablets: A randomized, single-dose, two-period crossover study in healthy Korean male volunteers
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam 13620, Korea.
- 3Department of Clinical Pharmacology and Therapeutics, Kyung Hee University College of Medicine and Hospital, Seoul 02447, Korea. bhkim98@khu.ac.kr
- KMID: 2351808
- DOI: http://doi.org/10.12793/tcp.2015.23.2.49
Abstract
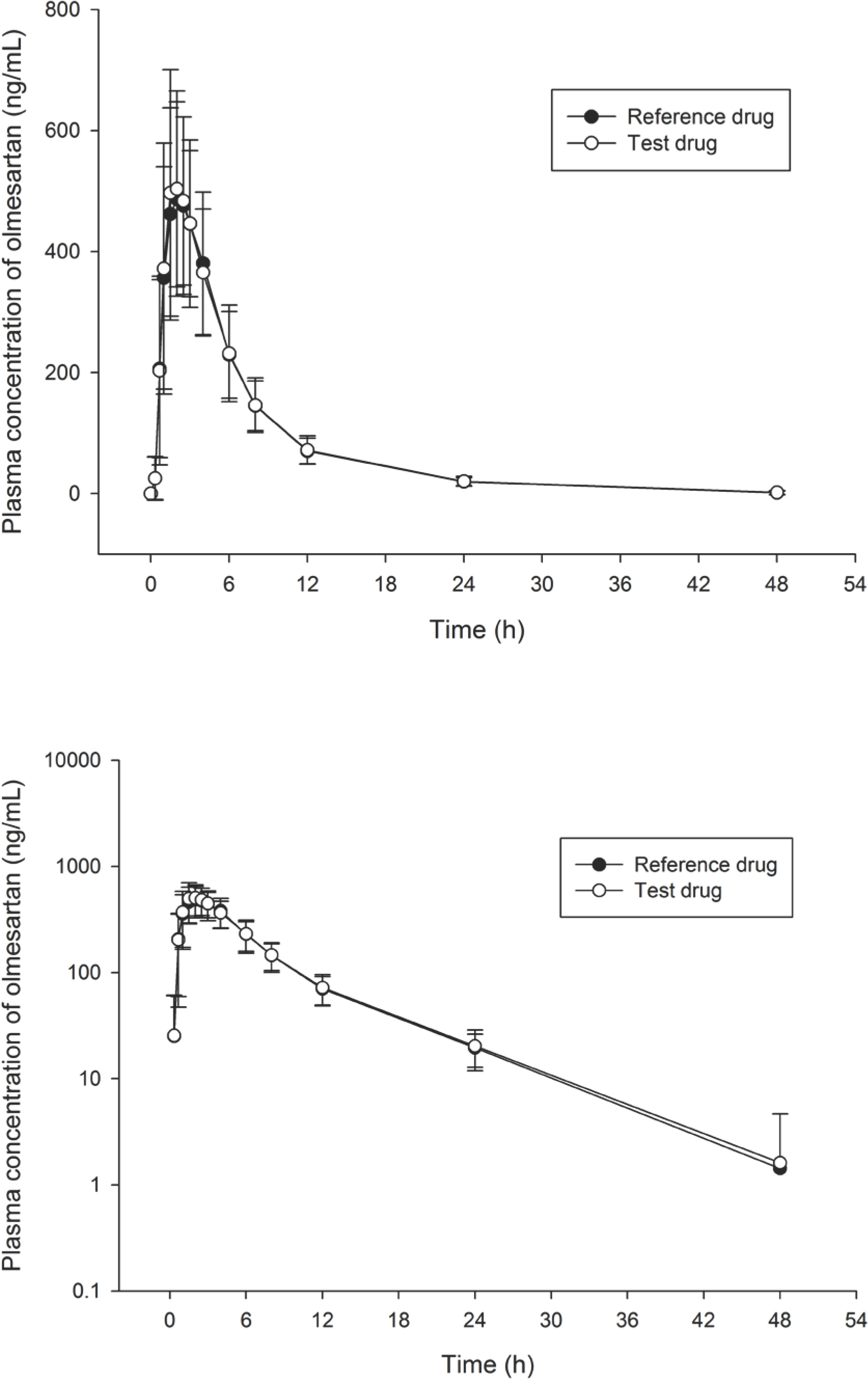
- Olmesartan is an angiotensin receptor blocker (ARB) and is widely used in clinical practice to treat hypertension. To compare the pharmacokinetic (PK) parameters and tolerability of two oral formulations of olmesartan (test drug: OMETAN(R) 20 mg tablet, reference drug: OLMETEC(R) 20 mg tablet) and assess their bioequivalence, a randomized, single dose, two-treatment crossover clinical study was conducted. At each period, 40 subjects received the test drug or the reference drug. Blood samples were collected at pre-dose and up to 48 h after study drug administration of each period. Plasma concentrations of olmesartan were measured using liquid chromatography-tandem mass spectrometry. To evaluate PK profiles, maximum plasma concentration (C(max)) and area under the concentration-time curve from zero to last measurable time (AUC(last)) were estimated using a non-compartmental method. Tolerability was evaluated based on the incidence of adverse events, vital signs, electrocardiograms, and laboratory tests. A total of 39 subjects completed the study. The geometric mean ratio and 90% confidence intervals (CI) of test drug to reference drug were 1.027 (0.969-1.088) for C(max) and 1.014 (0.957-1.074) for AUC(last), respectively. There were no serious adverse events and both formulations of olmesartan were well tolerated. The OMETAN 20 mg tablet was judged to be bioequivalent to the OLMETEC 20 mg tablet.
Keyword
MeSH Terms
Figure
Reference
-
References
1. von Bergmann K, Laeis P, Puchler K, Sudhop T, Schwocho LR, Gonzalez L. Olmesartan medoxomil: influence of age, renal and hepatic function on the pharmacokinetics of olmesartan medoxomil. J Hypertens Suppl. 2001; 19:S33–S40.
Article2. Nelson M. Drug treatment of elevated blood pressure. Aust Prescr. 2010; 33:108–112.
Article3. Jaques H. NICE guideline on hypertension. Eur Heart J. 2013; 34:406–408.4. Greathouse M. A review of olmesartan medoxomil monotherapy: antihypertensive efficacy similar to that of other angiotensin II receptor blocker/hydrochlorothiazide combinations? Congest Heart Fail. 2002; 8:313–320.
Article5. Jiang J, Liu D, Hu P. Pharmacokinetic and safety profile of olmesartan medoxomil in healthy Chinese subjects after single and multiple administrations. Pharmazie. 2009; 64:323–326.6. Rozza F, Trimarco V, Izzo R, Santoro M, Manzi MV, Marino M, et al. Antihypertensive response to combination of olmesartan and amlodipine does not depend on method and time of drug administration. High Blood Press Cardiovasc Prev. 2013; 20:25–32.
Article7. Domjan A, Kakuk P, Sandor J. The Helsinki Declaration at 50 years: comments on the 2013 modifications. Lege Artis Med. 2014; 24:152–158.8. Jemal M. High-throughput quantitative bioanalysis by LC/MS/MS. Biomed Chromatogr. 2000; 14:422–429.
Article9. Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004; 23:1921–1986.
Article10. Houin G. Bioequivalence studies: a new EMA guideline. Arzneimittelforschung. 2010; 60:169–170.
Article11. Yoshihara K, Gao Y, Shiga H, Wada DR, Hisaoka M. Population pharmacokinetics of olmesartan following oral administration of its prodrug, olmesartan medoxomil: in healthy volunteers and hypertensive patients. Clin Pharmacokinet. 2005; 44:1329–1342.12. Johns EJ. The neural regulation of the kidney in hypertension and renal failure. Exp Physiol. 2014; 99:289–294.
Article13. Monhart V. Hypertension and chronic renal insufficiency-chronic kidney failure. Vnitr Lek. 2003; 49:388–394.14. Volpe M, Tocci G. Olmesartan in the treatment of hypertension in elderly patients: a review of the primary evidence. Drugs Aging. 2013; 30:987–998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Pharmacokinetics and bioequivalence of two different 20 mg olmesartan tablets: A randomized, single-dose, two-period crossover study in healthy Korean male volunteers
- Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers
- Bioequivalence study of Donepezil hydrochloride in healthy Korean volunteers
- Bioequivalence and Dose Proportionality of Olmesartan Medoxomil Formulations
- Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects