Transl Clin Pharmacol.
2018 Mar;26(1):16-24. 10.12793/tcp.2018.26.1.16.
Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea.
- 2Research & Development Division, Alvogen Korea Co., Ltd. Seoul 07326, Republic of Korea.
- 3Center for Clinical Pharmacology and Biomedical Research Institute, Chonbuk National University Hospital, Jeonju 54907, Republic of Korea. mgkim@jbnu.ac.kr
- 4Department of Pharmacology, School of Medicine, Chonbuk National University, Jeonju 54907, Republic of Korea.
- KMID: 2420303
- DOI: http://doi.org/10.12793/tcp.2018.26.1.16
Abstract
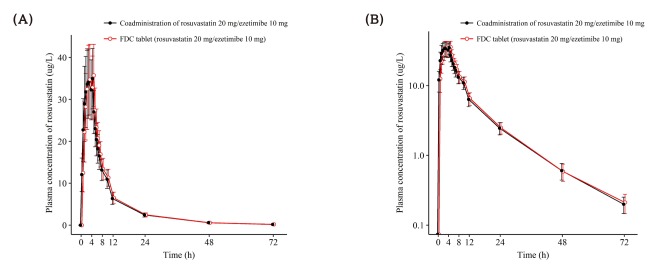
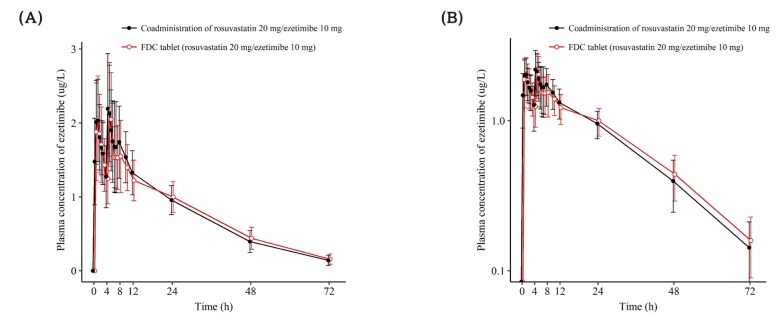
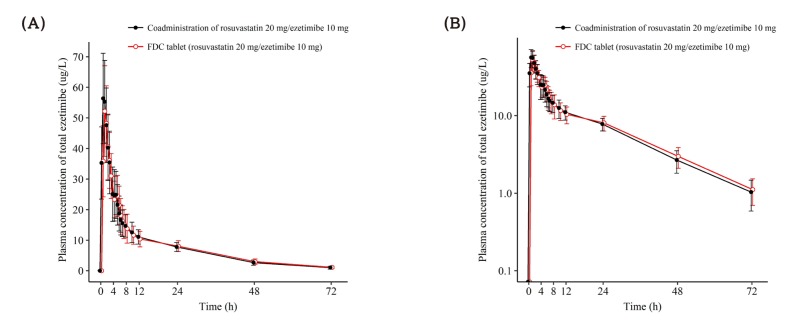
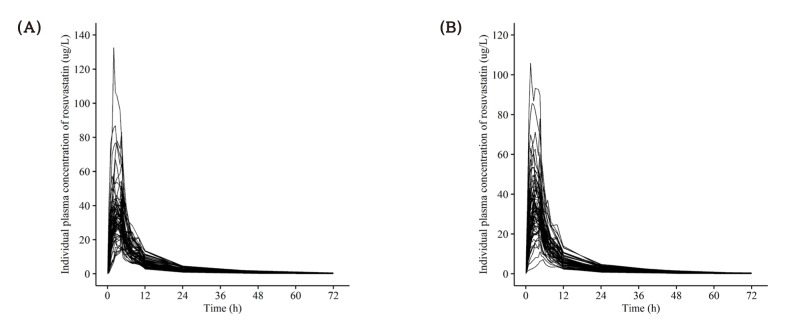
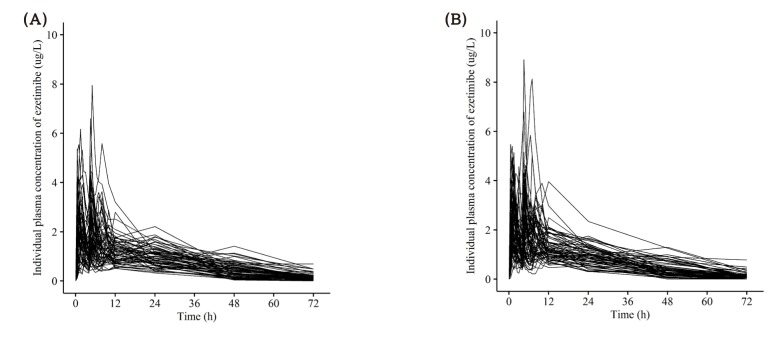
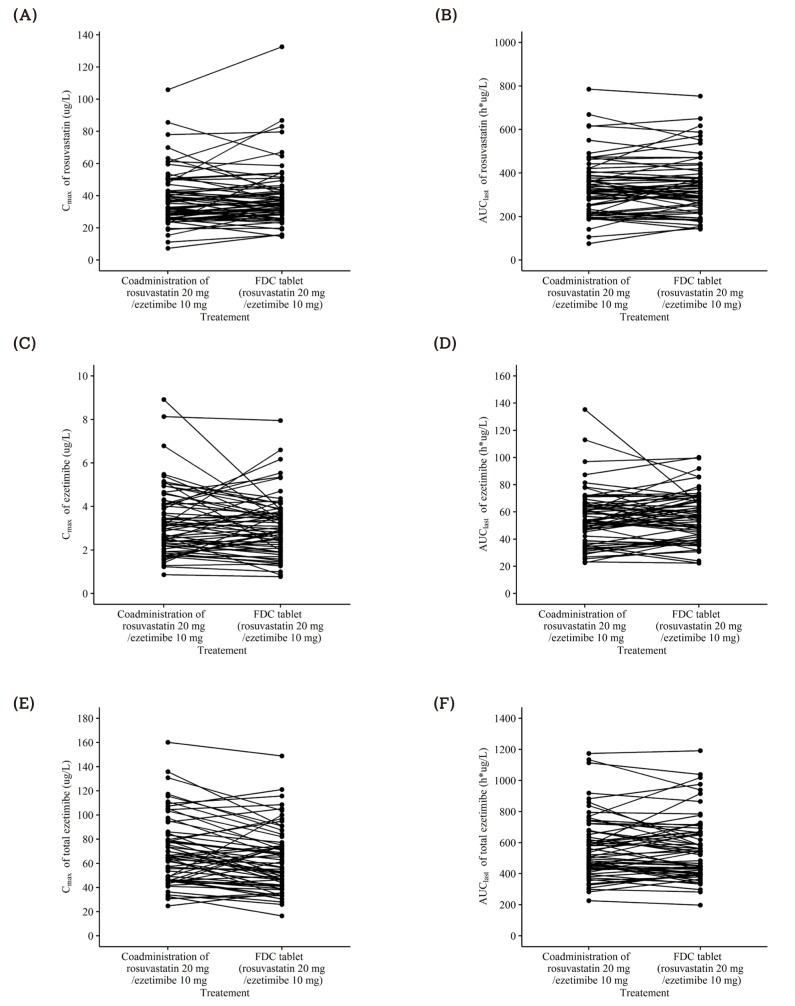
- This study aimed to compare the pharmacokinetics of fixed-dose combination (FDC) tablet of rosuvastatin 20 mg/ezetimibe 10 mg with that of concurrent administration of individual rosuvastatin 20 mg tablet and ezetimibe 10 mg tablet in healthy subjects. A randomized, open label, single-dose, two-way crossover study was conducted. Subjects randomly received test formulation (FDC tablet of rosuvastatin 20 mg/ezetimibe 10 mg) or reference formulation (co-administration of rosuvastatin 20 mg tablet and ezetimibe 10 mg tablet). After 2 weeks of washout, subjects received the other treatment. Blood samples were collected up to 72 hours post-dose in each period. Plasma concentrations of rosuvastatin, ezetimibe and total ezetimibe (ezetimibe + ezetimibe glucuronide) were analyzed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). The geometric mean ratio (GMR) of Cmax and AUClast (90% confidence interval, CI) for rosuvastatin was 1.036 (0.979-1.096) and 1.024 (0.981-1.070), respectively. The corresponding values for ezetimibe were 0.963 (0.888-1.043) and 1.021 (0.969-1.074), respectively. The corresponding values for total ezetimibe were 0.886 (0.835-0.940) and 0.983 (0.946-1.022), respectively. FDC tablet containing rosuvastatin 20 mg and ezetimibe 10 mg is bioequivalent to the co-administration of commercially available individual tablets of rosuvastatin and ezetimibe as GMR with 90% CI of Cmax and AUClast of rosuvastatin, ezetimibe and total ezetimibe were contained within conventionally accepted bioequivalence criteria.
MeSH Terms
Figure
Cited by 1 articles
-
Development and assessment of nano drug delivery systems for combined delivery of rosuvastatin and ezetimibe
Mohamed Ali Metwally, El-Yamani Ibrahim El-Zawahry, Maher Amer Ali, Diaa Farrag Ibrahim, Shereen Ahmed Sabry, Omnia Mohamed Sarhan
Korean J Physiol Pharmacol. 2024;28(3):275-284. doi: 10.4196/kjpp.2024.28.3.275.
Reference
-
1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129(Suppl 2):S1–S45. DOI: 10.1161/01.cir.0000437738.63853.7a.PMID: 24222016.2. Bullano MF1, Wertz DA, Yang GW, Kamat S, Borok GM, Gandhi S, et al. Effect of Rosuvastatin Compared with Other Statins on Lipid Levels and National Cholesterol Education Program Goal Attainment for Low-Density Lipoprotein Cholesterol in a Usual Care Setting. Pharmacotherapy. 2006; 26:469–478. PMID: 16553504.
Article3. Schuster H. The GALAXY Program: an update on studies investigating efficacy and tolerability of rosuvastatin for reducing cardiovascular risk. Expert Rev Cardiovasc Ther. 2007; 5:177–193. PMID: 17338663.
Article4. Waters DD, Brotons C, Chiang CW, Ferrières J, Foody J, Jukema JW, et al. Lipid treatment assessment project 2 : a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009; 120:28–34. DOI: 10.1161/CIRCULATIONAHA.108.838466. PMID: 19546386.5. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: A Review of its Metabolism, Pharmacokinetics and Drug Interactions. Clin Pharmacokinet. 2005; 44:467–494. PMID: 15871634.6. Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001; 23:1209–1230. PMID: 11558859.
Article7. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387–2397. DOI: 10.1056/NEJMoa1410489. PMID: 26039521.
Article8. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 20017; 120:713–719.
Article9. Trabelsi F, Bartůnek A, Vlavonou R, Navrátilová L, Dubé C, Tanguay M, et al. Single-dose, 2-way crossover, bioequivalence study of two rosuvastatin formulations in normal healthy subjects under fasting conditions. Int J Clin Pharmacol Ther. 2012; 50:741–750. DOI: 10.5414/CP201687. PMID: 22762855.
Article10. Migoya EM1, Bergman A, Hreniuk D, Matthews N, Yi B, Roadcap B, et al. Bioequivalence of an ezetimibe/simvastatin combination tablet and coadministration of ezetimibe and simvastatin as separate tablets in healthy subjects. Int J Clin Pharmacol Ther. 2006; 44:83–92. PMID: 16502768.
Article11. Crestor (rosuvastatin calcium) [package insert]. Wilmington, DE: Astra-Zeneca;2010.12. Zetia (ezetimibe) [package insert]. Whitehouse Station, NJ: Merck & CO., Inc.;2012.13. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016; 37:2999–3058. PMID: 27567407.
Article14. Kosoglou T, Statkevich P, Yang B, Suresh R, Zhu Y, Boutros T, et al. Pharma codynamic interaction between ezetimibe and rosuvastatin. Curr Med Res Opin. 2004; 20:1185–1195. PMID: 15324521.15. Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002; 41:751–790. PMID: 12162761.16. US Food and Drug AdministrationGuidance for industry. Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. 2014.17. Patrick JE, Kosoglou T, Stauber KL, Alton KB, Maxwell SE, Zhu Y, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos. 2002; 30:430–437. PMID: 11901097.
Article18. Davies NM, Takemoto JK, Brocks DR, Yáñez JA. Multiple peaking phenomena in pharmacokinetic disposition. Clin Pharmacokinet. 2010; 49:351–377. DOI: 10.2165/11319320-000000000-00000. PMID: 20481648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Pharmacokinetic Equivalence of the High Dose Strength Fixed-Dose Combination Tablet of Gemigliptin/Metformin Sustained Release (SR) and Individual Component Gemigliptin and Metformin XR Tablets in Healthy Subjects
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- Pharmacokinetics of atorvastatin and sustained-release metformin fixed-dose combination tablets: two randomized, open-label, 2-way crossover studies in healthy male subjects under fed conditions