Korean J Physiol Pharmacol.
2016 Sep;20(5):533-538. 10.4196/kjpp.2016.20.5.533.
Regulation of retinal angiogenesis by endothelial nitric oxide synthase signaling pathway
- Affiliations
-
- 1Gene and Cell Therapy Center for Vessel-Associated Disease, Medical Research Institute, and Department of Pharmacology, Pusan National University School of Medicine, Yangsan 50612, Korea. sunsik@pusan.ac.kr
- 2Department of Anatomy, Pusan National University of Korean Medicine, Yangsan 50612, Korea.
- 3Department of Obstetrics and Gynecology, Pusan National University Hospital, Yangsan 50612, Korea.
- 4Department of Internal Medicine, Pusan National University Hospital, Busan 49241, Korea.
- KMID: 2350510
- DOI: http://doi.org/10.4196/kjpp.2016.20.5.533
Abstract
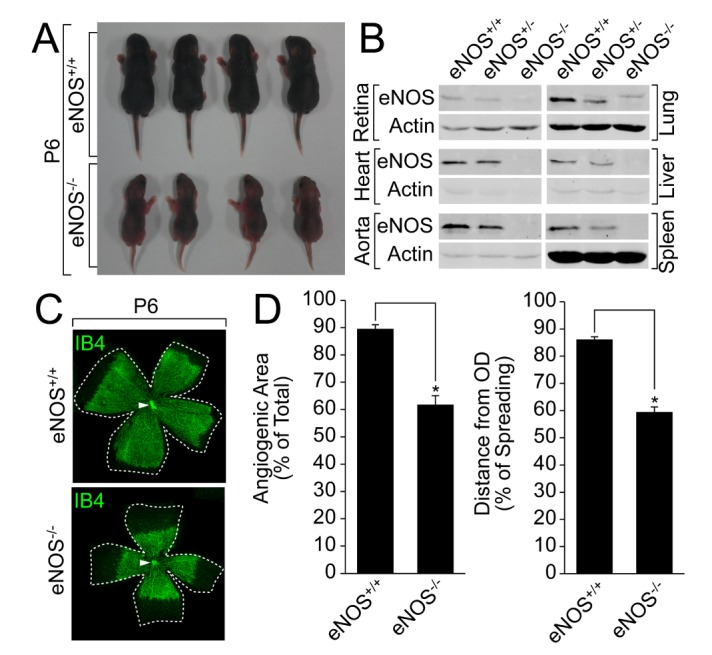
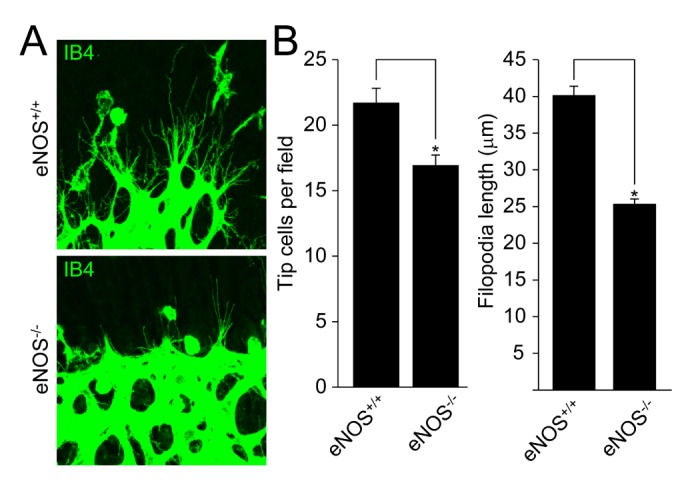
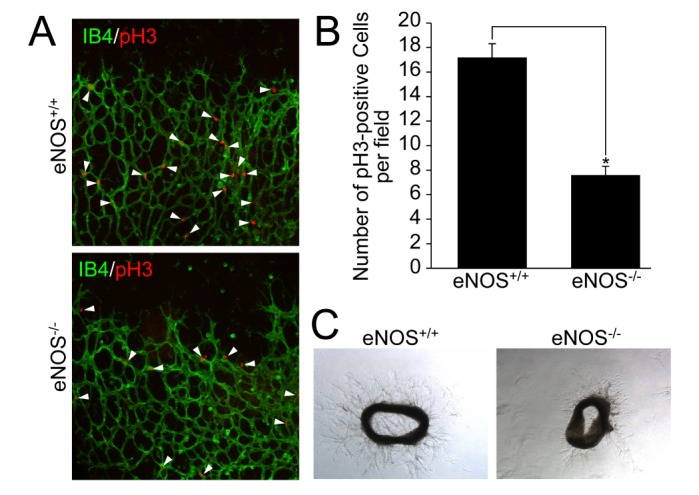
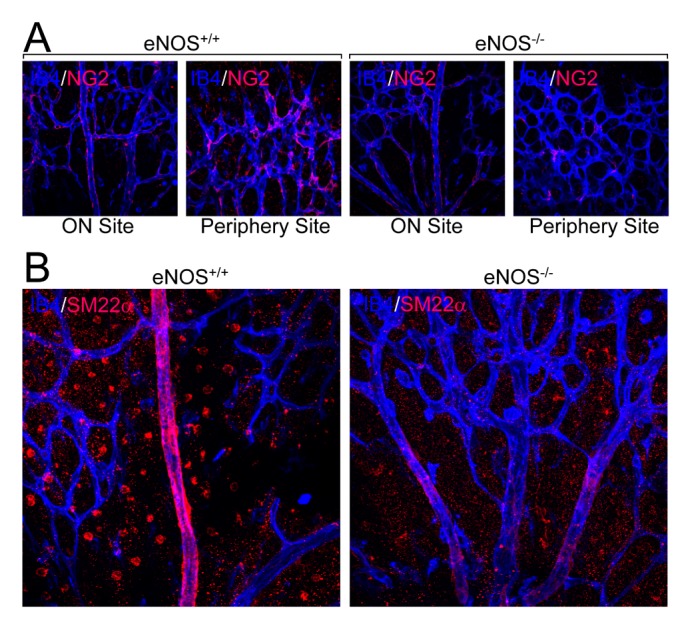
- Angiogenesis plays an essential role in embryo development, tissue repair, inflammatory diseases, and tumor growth. In the present study, we showed that endothelial nitric oxide synthase (eNOS) regulates retinal angiogenesis. Mice that lack eNOS showed growth retardation, and retinal vessel development was significantly delayed. In addition, the number of tip cells and filopodia length were significantly reduced in mice lacking eNOS. Retinal endothelial cell proliferation was significantly blocked in mice lacking eNOS, and EMG-2-induced endothelial cell sprouting was significantly reduced in aortic vessels isolated from eNOS-deficient mice. Finally, pericyte recruitment to endothelial cells and vascular smooth muscle cell coverage to blood vessels were attenuated in mice lacking eNOS. Taken together, we suggest that the endothelial cell function and blood vessel maturation are regulated by eNOS during retinal angiogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007; 100:782–794. PMID: 17395884.
Article2. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003; 161:1163–1177. PMID: 12810700.
Article3. Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51:2813–2826. PMID: 20484600.
Article4. Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006; 312:676–683. PMID: 16337189.
Article5. Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994; 78:915–918. PMID: 7522969.
Article6. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012; 33:829–837. 837a–837d. PMID: 21890489.7. Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009; 2:ra41. PMID: 19654415.
Article8. Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A. 2014; 111:12865–12870. PMID: 25136137.
Article9. Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005; 102:10999–11004. PMID: 16043715.
Article10. Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001; 98:2604–2609. PMID: 11226286.
Article11. Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996; 93:13176–13181. PMID: 8917564.
Article12. Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997; 100:3131–3139. PMID: 9399960.
Article13. Papapetropoulos A, García-Cardeña G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999; 79:213–223. PMID: 10068209.14. Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002; 16:706–708. PMID: 11978735.
Article15. Le Romancer M, Reyl-Desmars F, Cherifi Y, Pigeon C, Bottari S, Meyer O, Lewin MJ. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994; 269:17464–17468. PMID: 8021251.
Article16. Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994; 94:2036–2044. PMID: 7525653.
Article17. Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003; 9:1370–1376. PMID: 14556003.
Article18. Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998; 101:2567–2578. PMID: 9616228.
Article19. Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol. 2010; 135:247–259. PMID: 20176853.20. Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005; 115:1816–1827. PMID: 15951843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and Regulation of Endothelial Nitric Oxide Synthase by Vascular Endothelial Growth Factor in ECV 304 Cells
- Immunohistochemical Expression of Placental Nitric Oxide Synthase in Preeclampsia and Normal Pregnancy
- Effect and Mechanism of Vascular Endothelial Growth Factor on Endothelial Nitric Oxide Synthase Expression in Aortic Endothelial Cells
- Genetic Polymorphism of Endothelial Nitric Oxide Synthase in Korean Schizophrenic Patients
- Role of nitric oxide and distribution of nitric oxide synthase in the gustatory system