Using Periostin as a Biomarker in the Treatment of Asthma
- Affiliations
-
- 1Division of Medical Biochemistry, Department of Biomolecular Sciences, Saga Medical School, Saga, Japan. kizuhara@cc.saga-u.ac.jp
- 2Shino-test Co. Ltd., Sagamihara, Japan.
- KMID: 2349988
- DOI: http://doi.org/10.4168/aair.2016.8.6.491
Abstract
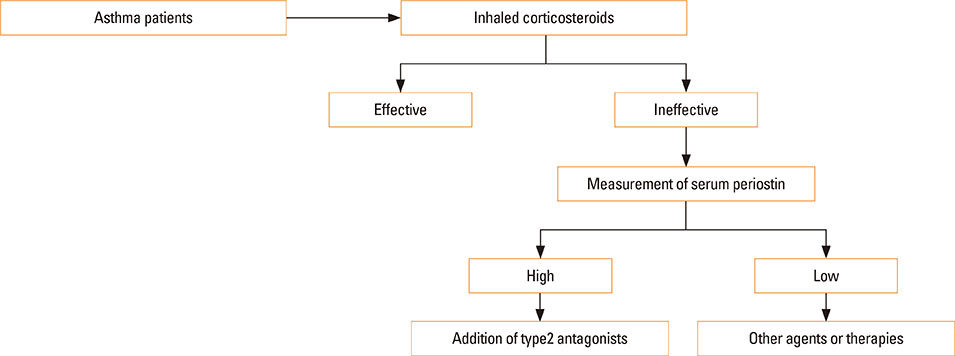
- Periostin acts both as an extracellular matrix protein belonging to the fasciclin family and as a matricellular protein functioning in cell activation by binding to its receptors on the cell surface. It has been established that periostin is a downstream molecule of interleukin (IL)-13, a signature type 2 cytokine, and that periostin plays an important role in the pathogenesis of allergic diseases, including asthma. Based on these findings, much attention has been paid to periostin as a biomarker useful in the treatment of asthma. Periostin is a surrogate biomarker for type 2 immunity; it has been shown that serum periostin can predict the efficacy of anti-IL-13 antibodies (lebrikizumab) and anti-IgE antibodies (omalizumab), and that this usefulness can be potentially expanded to other type 2 antagonists. Moreover, it has been shown that periostin is not a simple surrogate biomarker for type 2 immunity; periostin-high asthma patients have several unique characteristics, including eosinophilia, high fraction of nitric oxide, aspirin intolerance, nasal disorders, and late onset. These characteristics are likely to be correlated with the involvement of periostin in the tissue remodeling of asthma. Periostin is also associated with hyporesponsiveness to inhaled corticosteroids, probably reflecting tissue remodeling. Thus, periostin has 2 characteristics as a biomarker for early diagnosis of asthma: surrogate biomarkers for type 2 immunity and tissue remodeling. Based on these characteristics, we will be able to apply serum periostin to treatment of asthma.
MeSH Terms
Figure
Cited by 3 articles
-
Serum Periostin Is Negatively Correlated With Exposure to Formaldehyde and Volatile Organic Compounds in Children
Dong Keon Yon, Jaewoo An, Eun Kyo Ha, Hye Mi Jee, Kenji Izuhara, Junya Ono, Young-Ho Jung, Kyung Suk Lee, Youn Ho Sheen, Heysung Baek, Man Yong Han
Allergy Asthma Immunol Res. 2018;10(6):716-721. doi: 10.4168/aair.2018.10.6.716.Association Between Epithelial Cytokines and Clinical Phenotypes of Elderly Asthma
Bastsetseg Ulambayar, So-Hee Lee, Eun-Mi Yang, Young-Min Ye, Hae-Sim Park
Allergy Asthma Immunol Res. 2019;11(1):79-89. doi: 10.4168/aair.2019.11.1.79.Serum Periostin Levels: A Potential Serologic Marker for Toluene Diisocyanate-Induced Occupational Asthma
Ji-Ho Lee, Sang-Ha Kim, Youngwoo Choi, Hoang Kim Tu Trinh, Eun-Mi Yang, Ga-Young Ban, Yoo Seob Shin, Young-Min Ye, Kenji Izuhara, Hae-Sim Park
Yonsei Med J. 2018;59(10):1214-1221. doi: 10.3349/ymj.2018.59.10.1214.
Reference
-
1. Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014; 71:1279–1288.2. Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014; 63:143–151.3. Izuhara K, Matsumoto H, Ohta S, Ono J, Arima K, Ogawa M. Recent developments regarding periostin in bronchial asthma. Allergol Int. 2015; 64:Suppl. S3–S10.4. Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. Forthcoming 2016.5. Izuhara K, Arima K, Kanaji S, Ohta S, Kanaji T. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006; 13:2291–2298.6. Izuhara K, Ohta S, Shiraishi H, Suzuki S. Interleukin 4, interleukin 13, and interleukin 9. In : Izuhara K, Holgate ST, Wills-Karp M, editors. Inflammation and allergy drug design. London: Wiley-Blackwell;2011. p. 175–185.7. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263.8. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282:2258–2261.9. Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999; 103:779–788.10. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002; 8:885–889.11. Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002; 19:287–296.12. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; 118:98–104.13. Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-γ and IL-13 production. Proc Natl Acad Sci U S A. 2007; 104:14765–14770.14. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863.15. Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011; 186:4959–4966.16. Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, et al. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012; 42:144–155.17. Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, et al. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014; 134:1433–1442.18. Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013; 132:305–312.e3.19. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012; 122:2590–2600.20. Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012; 46:677–686.21. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.22. Willis JC, Lord GM. Immune biomarkers: the promises and pitfalls of personalized medicine. Nat Rev Immunol. 2015; 15:323–329.23. Hughes B. Developing tools for stratified medicine. Nat Rev Drug Discov. 2009; 8:919–920.24. Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007; 6:287–293.25. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.26. Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003; 167:1655–1659.27. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–1207.28. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.29. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431.30. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010; 181:788–796.31. Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011; 183:1007–1014.32. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.33. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.34. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.35. Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003; 178:347–355.36. Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985). 2008; 104:394–403.37. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008; 178:218–224.38. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.39. Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014; 113:314–320.40. Matsusaka M, Kabata H, Fukunaga K, Suzuki Y, Masaki K, Mochimaru T, et al. Phenotype of asthma related with high serum periostin levels. Allergol Int. 2015; 64:175–180.41. Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. Forthcoming 2016.42. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015; 70:115–120.43. Arron JR, Izuhara K. Asthma biomarkers: what constitutes a 'gold standard'? Thorax. 2015; 70:105–107.44. Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Horiguchi T, et al. Using exhaled nitric oxide and serum periostin as a composite marker to identify severe/steroid-insensitive asthma. Am J Respir Crit Care Med. 2014; 190:1449–1452.45. Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012; 61:589–595.46. Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, López-Carrasco V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015; 70:540–546.47. Hekking PP, Bel EH. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract. 2014; 2:671–680.48. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005; 172:149–160.49. Kanemitsu Y, Matsumoto H, Mishima M. KiHAC Respiratory Medicine Group. Factors contributing to an accelerated decline in pulmonary function in asthma. Allergol Int. 2014; 63:181–188.50. Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Kita H, et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy Clin Immunol. 2014; 133:1474–1477. 1477.e1–1477.e2.51. Kato G, Takahashi K, Izuhara K, Komiya K, Kimura S, Hayashi S. Markers that can reflect asthmatic activity before and after reduction of inhaled corticosteroids: a pilot study. Biomark Insights. 2013; 8:97–105.52. Song JS, You JS, Jeong SI, Yang S, Hwang IT, Im YG, et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy. 2015; 70:674–681.53. Konradsen JR, Skantz E, Nordlund B, Lidegran M, James A, Ono J, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol. 2015; 26:772–779.54. Inoue Y, Izuhara K, Ohta S, Ono J, Shimojo N. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol Int. 2015; 64:289–290.55. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015; 3:692–701.56. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.57. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366.58. Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879–890.59. Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014; 69:1223–1232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Periostin in Exhaled Breath Condensate and in Serum of Asthmatic Patients: Relationship to Upper and Lower Airway Disease
- Serum Periostin Levels: A Potential Serologic Marker for Toluene Diisocyanate-Induced Occupational Asthma
- Roles of Periostin in Symptom Manifestation and Airway Remodeling in a Murine Model of Allergic Rhinitis
- Clinical perspective on serum periostin in antineutrophil-cytoplasmic antibody-associated vasculitis
- Serum Levels of Eosinophil-Derived Neurotoxin: A Biomarker for Asthma Severity in Adult Asthmatics