Allergy Asthma Immunol Res.
2019 May;11(3):394-405. 10.4168/aair.2019.11.3.394.
Serum Levels of Eosinophil-Derived Neurotoxin: A Biomarker for Asthma Severity in Adult Asthmatics
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Pulmonology and Allergy, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Asthma and Allergy Center, Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 4Department of Allergy, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2442050
- DOI: http://doi.org/10.4168/aair.2019.11.3.394
Abstract
- PURPOSE
Eosinophilic inflammation is a key component of severe asthma (SA). However, there has been no reliable serum biomarker for the eosinophilic inflammation of SA. We hypothesized that serum eosinophil-derived neurotoxin (EDN) could predict the eosinophilic inflammation of SA in adult asthmatics.
METHODS
Severe asthmatics (n = 235), nonsevere asthmatics (n = 898), and healthy controls (n = 125) were enrolled from Ajou University Hospital, South Korea. The serum levels of EDN and periostin were measured by enzyme-linked immunosorbent assay and compared between severe and nonsevere asthmatics. Their associations with total eosinophil count (TEC) and clinical parameters were evaluated; clinical validation of the K-EDN kit for the measurement of serum EDN was evaluated.
RESULTS
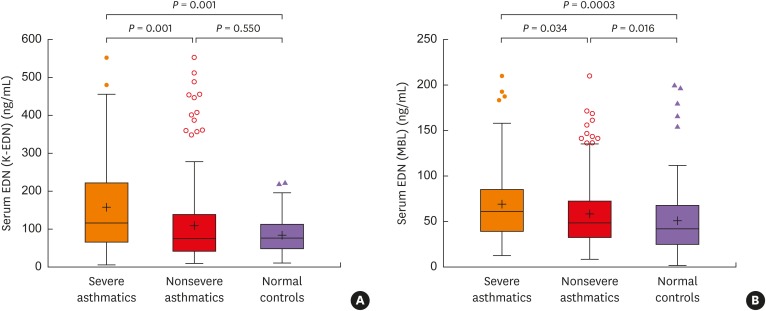
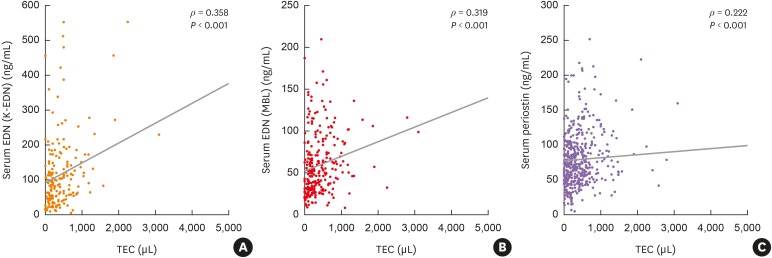
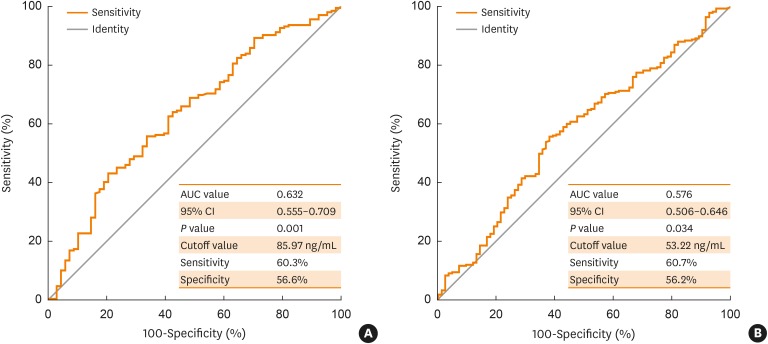
Severe asthmatics were older and had longer disease duration with significantly lower levels of forced expiratory volume in 1 second and methacholine PC20 than nonsevere asthmatics. Significant differences were found in TEC or sputum eosinophil count (%) between the groups. The serum levels of EDN and periostin were significantly higher in severe asthmatics than in nonsevere asthmatics and in healthy controls (all P < 0.05). Although significant correlations were found between serum EDN levels measured by the 2 kits (Ï = 0.545, P < 0.0001), higher correlation coefficients between serum EDN levels measured by the K-EDN kit and TEC were higher (Ï = 0.358, P < 0.0001) than those between serum EDN levels measured by the MBL kit and TEC (Ï = 0.319, P < 0.0001) or serum periostin level (Ï = 0.222, P < 0.0001). Multivariate regression analysis demonstrated that serum EDN levels measured by the K-EDN kit predicted the phenotype of SA (P = 0.003), while 2 other biomarkers did not.
CONCLUSIONS
The serum EDN level may be a useful biomarker for assessing asthma severity in adult asthmatics.
Keyword
MeSH Terms
Figure
Reference
-
1. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534. PMID: 27582404.
Article2. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366. PMID: 25736990.
Article3. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659. PMID: 22901886.
Article4. Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014; 113:314–320. PMID: 25037608.
Article5. Pin I, Freitag AP, O'Byrne PM, Girgis-Gabardo A, Watson RM, Dolovich J, et al. Changes in the cellular profile of induced sputum after allergen-induced asthmatic responses. Am Rev Respir Dis. 1992; 145:1265–1269. PMID: 1595989.
Article6. Pizzichini MM, Pizzichini E, Clelland L, Efthimiadis A, Mahony J, Dolovich J, et al. Sputum in severe exacerbations of asthma: kinetics of inflammatory indices after prednisone treatment. Am J Respir Crit Care Med. 1997; 155:1501–1508. PMID: 9154849.
Article7. Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001; 119:1322–1328. PMID: 11348935.
Article8. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015; 70:115–120. PMID: 25422384.9. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013; 132:72–80. PMID: 23706399.
Article10. Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013; 13:11. PMID: 23442497.
Article11. Goldman M, Hirsch I, Zangrilli JG, Newbold P, Xu X. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the Phase III SIROCCO and CALIMA studies. Curr Med Res Opin. 2017; 33:1605–1613. PMID: 28644104.
Article12. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016; 150:799–810. PMID: 27018175.13. Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016; 4:549–556. PMID: 27177493.
Article14. Kim CK. Eosinophil-derived neurotoxin: a novel biomarker for diagnosis and monitoring of asthma. Korean J Pediatr. 2013; 56:8–12. PMID: 23390439.
Article15. Taniuchi S, Chihara J, Kojima T, Yamamoto A, Sasai M, Kobayashi Y. Serum eosinophil derived neurotoxin may reflect more strongly disease severity in childhood atopic dermatitis than eosinophil cationic protein. J Dermatol Sci. 2001; 26:79–82. PMID: 11323224.
Article16. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373. PMID: 24337046.17. Kim SH, Yang EM, Lee HN, Choi GS, Ye YM, Park HS. Association of the CCR3 gene polymorphism with aspirin exacerbated respiratory disease. Respir Med. 2010; 104:626–632. PMID: 20022477.
Article18. Kim CK, Callaway Z, Park JS, Kwon E. Utility of serum eosinophil-derived neurotoxin (EDN) measurement by ELISA in young children with asthma. Allergol Int. 2017; 66:70–74. PMID: 27329145.
Article19. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012; 122:2590–2600. PMID: 22684102.
Article20. Parulekar AD, Atik MA, Hanania NA. Periostin, a novel biomarker of TH2-driven asthma. Curr Opin Pulm Med. 2014; 20:60–65. PMID: 24247042.
Article21. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098. PMID: 21812663.
Article22. Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013; 131:1504–1512. PMID: 23506843.23. Kim CK, Callaway Z, Fletcher R, Koh YY. Eosinophil-derived neurotoxin in childhood asthma: correlation with disease severity. J Asthma. 2010; 47:568–573. PMID: 20560830.
Article24. European Network for Understanding Mechanisms of Severe Asthma. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003; 22:470–477. PMID: 14516137.25. Dolan CM, Fraher KE, Bleecker ER, Borish L, Chipps B, Hayden ML, et al. Design and baseline characteristics of the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study: a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2004; 92:32–39. PMID: 14756462.
Article26. Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007; 119:405–413. PMID: 17291857.
Article27. Belda J, Leigh R, Parameswaran K, O'Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. 2000; 161:475–478. PMID: 10673188.
Article28. Spanevello A, Confalonieri M, Sulotto F, Romano F, Balzano G, Migliori GB, et al. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med. 2000; 162:1172–1174. PMID: 10988149.29. Thomas RA, Green RH, Brightling CE, Birring SS, Parker D, Wardlaw AJ, et al. The influence of age on induced sputum differential cell counts in normal subjects. Chest. 2004; 126:1811–1814. PMID: 15596678.
Article30. Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol. 2014; 113:19–24. PMID: 24846699.
Article31. Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 2013; 68:402–406. PMID: 23347007.
Article32. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323. PMID: 19892860.
Article33. Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014; 133:1280–1288. PMID: 24589344.
Article34. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008; 178:218–224. PMID: 18480428.
Article35. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002; 360:1715–1721. PMID: 12480423.
Article36. Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006; 27:483–494. PMID: 16507847.
Article37. Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006; 34:129–139. PMID: 16749408.
Article38. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61. PMID: 16423202.
Article39. Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013; 62:1395–1405. PMID: 22895393.
Article40. Gon Y, Ito R, Hattori T, Hiranuma H, Kumasawa F, Kozu Y, et al. Serum eosinophil-derived neurotoxin: correlation with persistent airflow limitation in adults with house-dust mite allergic asthma. Allergy Asthma Proc. 2015; 36:e113–20. PMID: 26534742.
Article41. Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018; 50:104. PMID: 30115903.
Article42. Kim KW, Lee KE, Kim ES, Song TW, Sohn MH, Kim KE. Serum eosinophil-derived neurotoxin (EDN) in diagnosis and evaluation of severity and bronchial hyperresponsiveness in childhood asthma. Lung. 2007; 185:97–103. PMID: 17393238.
Article43. Wojnarowski C, Roithner B, Koller DY, Halmerbauer G, Gartner C, Tauber E, et al. Lack of relationship between eosinophil cationic protein and eosinophil protein X in nasal lavage and urine and the severity of childhood asthma in a 6-month follow-up study. Clin Exp Allergy. 1999; 29:926–932. PMID: 10383593.
Article44. Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med. 2000; 161:1185–1190. PMID: 10764310.45. Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013; 132:297–304.e3. PMID: 23683514.
Article46. Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014; 134:1175–1186.e7. PMID: 25042748.47. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135. PMID: 17083726.
Article48. Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015; 7:301ra129.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eosinophil-derived neurotoxin: a novel biomarker for diagnosis and monitoring of asthma
- The Association of Eosinophilic Airway Inflammation in Mycoplasma pneumonia and Asthma
- Marked Deposition of Eosinophil-Derived Neurotoxin in Adult Patients With Eosinophilic Esophagitis (Am J Gastroenterol 2010;105:298-307)
- Association between specific IgE to staphylococcal enterotoxin B and the eosinophilic phenotype of asthma
- The Efficacy of Eosinophilic Degranulation Proteins for Diagnosing Asthma in Chronic Cough Patients