Periostin in Exhaled Breath Condensate and in Serum of Asthmatic Patients: Relationship to Upper and Lower Airway Disease
- Affiliations
-
- 1Department of Clinical Immunology, Rheumatology and Allergy, Healthy Ageing Research Centre, Medical University of Lodz, Poland. marek.kowalski@csk.umed.lodz.pl
- 2Department of Rheumatology, Medical University of Lodz, Poland.
- KMID: 2413386
- DOI: http://doi.org/10.4168/aair.2017.9.2.126
Abstract
- PURPOSE
Periostin is considered a biomarker for eosinophilic airway inflammation and have been associated with NSAID-Exacerbated Respiratory Disease (NERD) and chronic rhinosinusitis (CRS). In this study, we aimed to evaluate periostin in exhaled breath condensate (EBC) and in serum of patients with various asthma phenotypes.
METHODS
The study included 40 asthmatic patients (22 with NERD) and 17 healthy controls. All the procedures (questionnaire, spirometry, FeNO, nasal swabs, EBC collecting, and blood sampling) were performed on the same day. Periostin concentrations were measured using an ELISA kit.
RESULTS
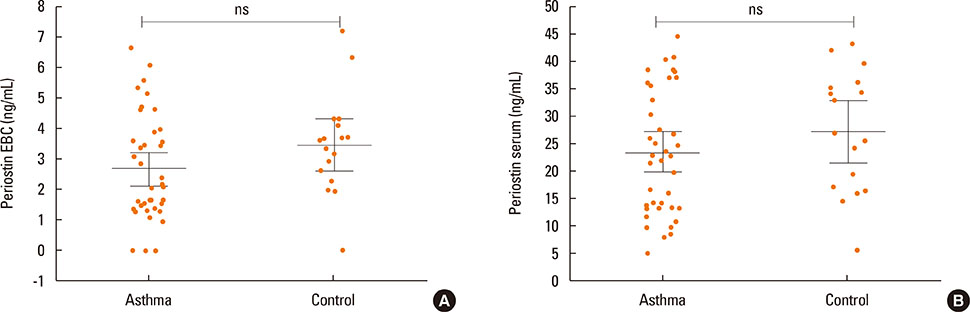
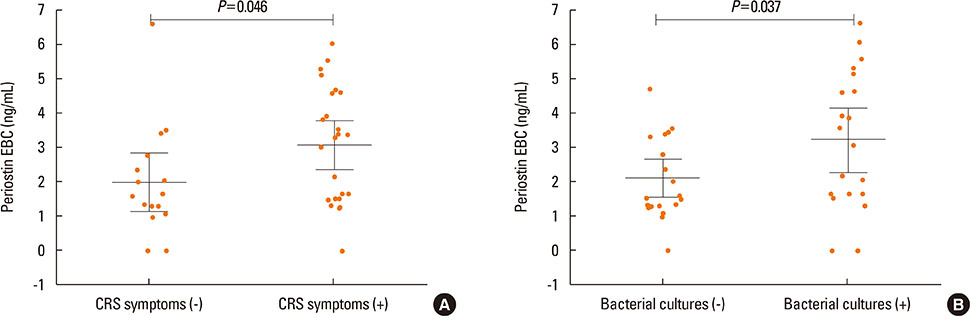
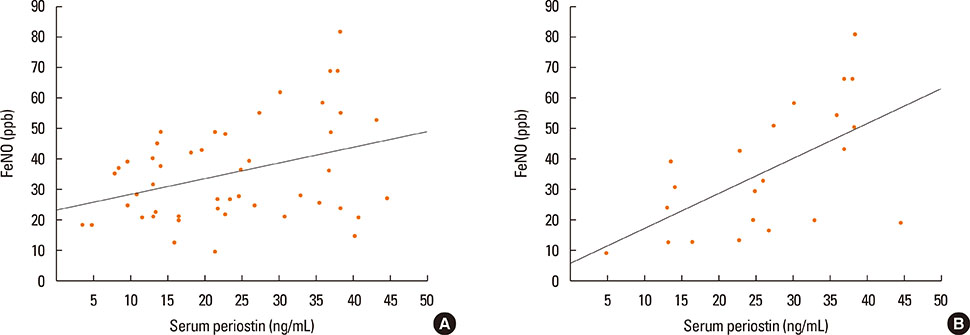
Periostin was detected in EBC from 37 of 40 asthmatics and in 16 from 17 of controls. The concentration of periostin in EBC did not differ between the study groups and was not associated with NERD or asthma severity. However, the EBC periostin was significantly higher in asthmatics with CRS as compared to those without (3.1 vs 2 ng/mL, P=0.046). Patients with positive bacterial culture from nasal swabs had higher EBC periostin concentrations than those without (3.2 vs 2.1 ng/mL; P=0.046). The mean serum periostin level was higher in asthmatics with a 1-year history of exacerbation than in those without (3.2 vs 2.3 ng/mL, P=0.045). Asthmatics with skin manifestation of NSAIDs hypersensitivity had higher serum periostin levels as compared to those without (3.5 vs 2.3 ng/mL; P=0.03).
CONCLUSIONS
EBC periostin levels seem to reflect intensity of upper airway disease in asthmatics, while serum levels of periostin are associated with asthma activity (exacerbations or FeNO) or NERD subphenotypes.
MeSH Terms
Figure
Cited by 3 articles
-
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
Wan Yin Winnie Yeung, Hae Sim Park
Yonsei Med J. 2020;61(1):4-14. doi: 10.3349/ymj.2020.61.1.4.Serum Periostin Is Negatively Correlated With Exposure to Formaldehyde and Volatile Organic Compounds in Children
Dong Keon Yon, Jaewoo An, Eun Kyo Ha, Hye Mi Jee, Kenji Izuhara, Junya Ono, Young-Ho Jung, Kyung Suk Lee, Youn Ho Sheen, Heysung Baek, Man Yong Han
Allergy Asthma Immunol Res. 2018;10(6):716-721. doi: 10.4168/aair.2018.10.6.716.Serum Periostin Levels: A Potential Serologic Marker for Toluene Diisocyanate-Induced Occupational Asthma
Ji-Ho Lee, Sang-Ha Kim, Youngwoo Choi, Hoang Kim Tu Trinh, Eun-Mi Yang, Ga-Young Ban, Yoo Seob Shin, Young-Min Ye, Kenji Izuhara, Hae-Sim Park
Yonsei Med J. 2018;59(10):1214-1221. doi: 10.3349/ymj.2018.59.10.1214.
Reference
-
1. Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014; 71:1279–1288.2. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863.3. Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012; 61:589–595.4. Kanemitsu Y, Matsumoto H, Mishima M. iHAC Respiratory Medicine Group. Factors contributing to an accelerated decline in pulmonary function in asthma. Allergol Int. 2014; 63:181–188.5. Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012; 130:647–654.e10.6. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015; 70:115–120.7. Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Kita H, et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy Clin Immunol. 2014; 133:1474–1477. 1477.e1–1477.e2.8. Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014; 113:314–320.9. Yamaguchi Y. Periostin in skin tissue and skin-related diseases. Allergol Int. 2014; 63:161–170.10. Ahmadzai H, Huang S, Hettiarachchi R, Lin JL, Thomas PS, Zhang Q. Exhaled breath condensate: a comprehensive update. Clin Chem Lab Med. 2013; 51:1343–1361.11. Konstantinidi EM, Lappas AS, Tzortzi AS, Behrakis PK. Exhaled breath condensate: technical and diagnostic aspects. ScientificWorldJournal. 2015; 2015:435160.12. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2011 [Internet]. [place unknown]: Global Initiative for Asthma;2011. cited 2016 Sep 16. Available from: http://www.ginasthma.org/.13. Lee RU, Stevenson DD. Aspirin-exacerbated respiratory disease: evaluation and management. Allergy Asthma Immunol Res. 2011; 3:3–10.14. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.15. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000; 162:2341–2351.16. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.17. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.18. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.19. Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005; 26:523–548.20. Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, López-Carrasco V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015; 70:540–546.21. Fingleton J, Travers J, Bowles D, Strik R, Siebers R, Holweg C, et al. Serum periostin in obstructive airways disease: range, relationships and response to corticosteroid. Eur Respir J. 2014; 44:P3873.22. James A, Ono J, Kupczyk M, Ohta S, Izuhara K, Dahlen SK. Controlled oral steroid intervention decreases serum periostin levels in asthmatic patients. Am J Respir Crit Care Med. 2013; 187:A6005.23. Simpson J, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Sputum and serum periostin levels are associated with, but do not predict sputum eosinophil proportion in severe asthma. Am J Respir Crit Care Med. 2015; 191:A4191.24. Seshadri S, Lu X, Purkey MR, Homma T, Choi AW, Carter R, et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015; 136:1548–1558. 1558.e1–1558.e7.25. Laury AM, Hilgarth R, Nusrat A, Wise SK. Periostin and receptor activator of nuclear factor κ-B ligand expression in allergic fungal rhinosinusitis. Int Forum Allergy Rhinol. 2014; 4:716–724.26. Thomson N, Chaudhuri R, Spears M, Nocka K, Jelinsky S, Miele G, et al. Serum periostin but not airway POSTN expression is reduced in smokers with asthma. Eur Respir J. 2013; 42:392.27. Hamilos DL. Drivers of chronic rhinosinusitis: Inflammation versus infection. J Allergy Clin Immunol. 2015; 136:1454–1459.28. Scichilone N, Crimi C, Benfante A, Battaglia S, Pitriolo P, Spatafora M, et al. Serum levels of periostin are higher in asthmatic with frequent exacerbations: a pilot study. Am J Respir Crit Care Med. 2015; 191:A4303.29. Nakamura Y, Nagashima H, Ohta S, Ono J, Yamauchi K, Izuhara K. Periostin in the bronchial lavage fluid of asthma patients. Allergol Int. 2015; 64:209–210.30. Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014; 133:98–103.e1-6. 103.e1–103.e6.31. Ledford DK, Wenzel SE, Lockey RF. Aspirin or other nonsteroidal inflammatory agent exacerbated asthma. J Allergy Clin Immunol Pract. 2014; 2:653–657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased inflammatory mediator in exhaled breath condensate from asthmatic children
- Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New?
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- Analysis of clinical features of adult asthma according to blood basophils and their association with cytokines in exhaled breath condensate
- Malondialdehyde and 3-Nitrotyrosine in Exhaled Breath Condensate in Retired Elderly Coal Miners with Chronic Obstructive Pulmonary Disease