Allergy Asthma Immunol Res.
2016 Nov;8(6):481-490. 10.4168/aair.2016.8.6.481.
Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New?
- Affiliations
-
- 1Department of Internal Medicine, Jagiellonian University Medical College, Krakow, Poland. nfsanak@cyf-kr.edu.pl
- KMID: 2349987
- DOI: http://doi.org/10.4168/aair.2016.8.6.481
Abstract
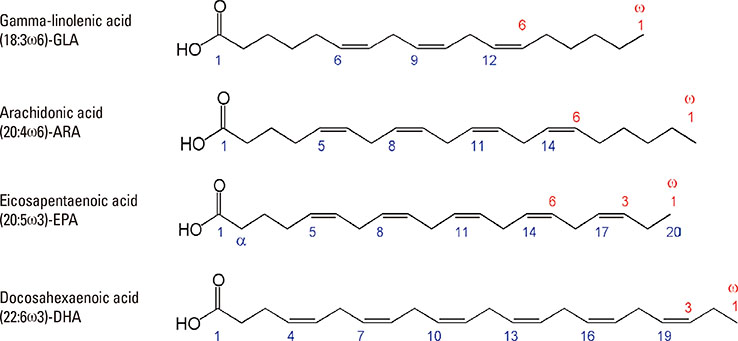
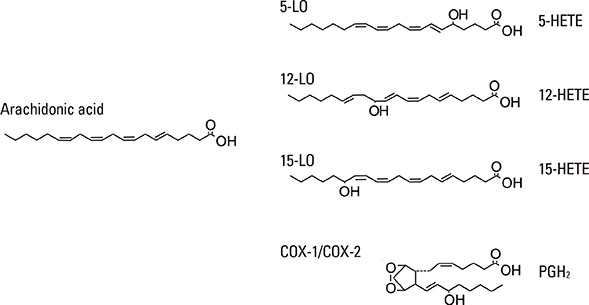
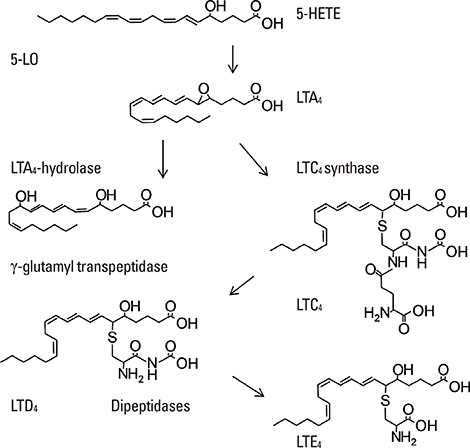
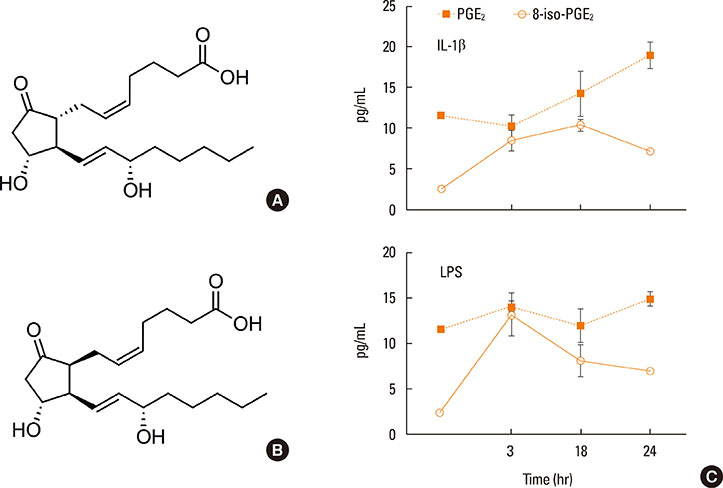
- Lipid mediators contribute to inflammation providing both pro-inflammatory signals and terminating the inflammatory process by activation of macrophages. Among the most significant biologically lipid mediators, these are produced by free-radical or enzymatic oxygenation of arachidonic acid named "eicosanoids". There were some novel eicosanoids identified within the last decade, and many of them are measurable in clinical samples by affordable chromatography-mass spectrometry equipment or sensitive immunoassays. In this review, we present some recent advances in understanding of the signaling by eicosanoid mediators during asthmatic airway inflammation. Eicosanoid profiling in the exhaled breath condensate, induced sputum, or their metabolites measurements in urine is complementary to the cellular phenotyping of asthmatic inflammation. Special attention is paid to aspirin-exacerbated respiratory disease, a phenotype of asthma manifested by the most profound changes in the profile of eicosanoids produced. A hallmark of this type of asthma with hypersensitivity to non-steroid anti-inflammatory drugs (NSAIDs) is to increase biosynthesis of cysteinyl leukotrienes on the systemic level. It depends on transcellular biosynthesis of leukotriene C₄ by platelets that adhere to granulocytes releasing leukotriene A₄. However, other abnormalities are also reported in this type of asthma as a resistance to anti-inflammatory activity of prostaglandin E₂ or a robust eosinophil interferon-γ response resulting in cysteinyl leukotrienes production. A novel mechanism is also discussed in which an isoprostane structurally related to prostaglandin E₂ is released into exhaled breath condensate during a provoked asthmatic attack. However, it is concluded that any single eicosanoid or even their complex profile can hardly provide a thorough explanation for the mechanism of asthmatic inflammation.
MeSH Terms
Figure
Reference
-
1. Shiraki A, Kume H, Oguma T, Makino Y, Ito S, Shimokata K, et al. Role of Ca2+ mobilization and Ca2+ sensitization in 8-iso-PGF2 alpha-induced contraction in airway smooth muscle. Clin Exp Allergy. 2009; 39:236–245.2. Horn T, Reddy Kakularam K, Anton M, Richter C, Reddanna P, Kuhn H. Functional characterization of genetic enzyme variations in human lipoxygenases. Redox Biol. 2013; 1:566–577.3. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002; 82:131–185.4. Brunnström Å, Tryselius Y, Feltenmark S, Andersson E, Leksell H, James A, et al. On the biosynthesis of 15-HETE and eoxin C4 by human airway epithelial cells. Prostaglandins Other Lipid Mediat. 2015; 121:83–90.5. Pace-Asciak CR. Pathophysiology of the hepoxilins. Biochim Biophys Acta. 2015; 1851:383–396.6. Reinauer C, Censarek P, Kaber G, Weber AA, Steger G, Klamp T, et al. Expression and translation of the COX-1b gene in human cells--no evidence of generation of COX-1b protein. Biol Chem. 2013; 394:753–760.7. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014; 510:92–101.8. Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci U S A. 2014; 111:E4753–E4761.9. Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue-regenerative resolvin and protectinsulfido-conjugated pathways. FASEB J. 2015; 29:2120–2136.10. Shinohara M, Mirakaj V, Serhan CN. Functional metabolomics reveals novel active products in the DHA metabolome. Front Immunol. 2012; 3:81.11. Howlett AC. A short guide to the nomenclature of seven-transmembrane spanning receptors for lipid mediators. Life Sci. 2005; 77:1522–1530.12. Petri MH, Thul S, Ovchinnikova O, Bäck M. Differential regulation of monocytic expression of leukotriene and lipoxin receptors. Prostaglandins Other Lipid Mediat. 2015; 121:138–143.13. Jakiela B, Gielicz A, Plutecka H, Hubalewska M, Mastalerz L, Bochenek G, et al. Eicosanoid biosynthesis during mucociliary and mucous metaplastic differentiation of bronchial epithelial cells. Prostaglandins Other Lipid Mediat. 2013; 106:116–123.14. Hiemstra PS, Mc Cray PB Jr, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. EurRespir J. 2015; 45:1150–1162.15. Pierzchalska M, Szabó Z, Sanak M, Soja J, Szczeklik A. Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J Allergy Clin Immunol. 2003; 111:1041–1048.16. Deacon K, Knox AJ. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE2, inducing COX-2, CXCL8, and VEGF expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015; 309:L237–L249.17. Mastalerz L, Sanak M, Kumik J, Gawlewicz-Mroczka A, Celejewska-Wójcik N, Cmiel A, et al. Exhaled eicosanoids following bronchial aspirin challenge in asthma patients with and without aspirin hypersensitivity: The pilot study. J Allergy (Cairo). 2012; 2012:696792.18. Choi GS, Kim JH, Shin YS, Ye YM, Kim SH, Park HS. Eosinophil activation and novel mediators in the aspirin-induced nasal response in AERD. Clin Exp Allergy. 2013; 43:730–740.19. Campo P, Ayuso P, Salas M, Plaza MC, Cornejo-García JA, Doña I, et al. Mediator release after nasal aspirin provocation supports different phenotypes in subjects with hypersensitivity reactions to NSAIDs. Allergy. 2013; 68:1001–1007.20. Bood JR, Sundblad BM, Delin I, Sjödin M, Larsson K, Anderson SD, et al. Urinary excretion of lipid mediators in response to repeated eucapnic voluntary hyperpnea in asthmatic subjects. J Appl Physiol (1985). 2015; 119:272–279.21. Bikov A, Gajdócsi R, Huszár É, Szili B, Lázár Z, Antus B, et al. Exercise increases exhaled breath condensate cysteinyl leukotriene concentration in asthmatic patients. J Asthma. 2010; 47:1057–1062.22. Baek HS, Cho J, Kim JH, Oh JW, Lee HB. Ratio of leukotriene e(4) to exhaled nitric oxide and the therapeutic response in children with exercise-induced bronchoconstriction. Allergy Asthma Immunol Res. 2013; 5:26–33.23. Hallstrand TS. New insights into pathogenesis of exercise-induced bronchoconstriction. Curr Opin Allergy Clin Immunol. 2012; 12:42–48.24. Higashi N, Taniguchi M, Mita H, Kawagishi Y, Ishii T, Higashi A, et al. Clinical features of asthmatic patients with increased urinary leukotriene E4 excretion (hyperleukotrienuria): involvement of chronic hyperplastic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2004; 113:277–283.25. Mastalerz L, Celejewska-Wójcik N, Wójcik K, Gielicz A, Januszek R, Cholewa A, et al. Induced sputum eicosanoids during aspirin bronchial challenge of asthmatic patients with aspirin hypersensitivity. Allergy. 2014; 69:1550–1559.26. Butterfield JH. Increased leukotriene E4 excretion in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 2010; 92:73–76.27. Maher SA, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Bonvini SJ, et al. Prostaglandin D2 and the role of the DP1, DP2 and TP receptors in the control of airway reflex events. Eur Respir J. 2015; 45:1108–1118.28. Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014; 133:899–901.e3.29. Powell WS, Rokach J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog Lipid Res. 2013; 52:651–665.30. Cosette C, Gravel S, Reddy CN, Gore V, Chourey S, Ye Q, et al. Biosynthesis and actions of 5-oxo eicosatetraenoic acid (5-oxo-ETE) on feline granulocytes. Biochem Pharmacol. 2015; 96:247–255.31. Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009; 206:2543–2555.32. Foster HR, Fuerst E, Lee TH, Cousins DJ, Woszczek G. Characterisation of P2Y(12) receptor responsiveness to cysteinyl leukotrienes. PLoS One. 2013; 8:e58305.33. Di Capite J, Shirley A, Nelson C, Bates G, Parekh AB. Intercellular Ca2+ wave propagation involving positive feedback between CRAC channels and cysteinyl leukotrienes. FASEB J. 2009; 23:894–905.34. Kupczyk M, Dahlen B, Sterk PJ, Nizankowska-Mogilnicka E, Papi A, Bel EH, et al. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014; 69:1198–1204.35. Mastalerz L, Celejewska-Wójcik N, Wójcik K, Gielicz A, Ćmiel A, Ignacak M, et al. Induced sputum supernatant bioactive lipid mediators can identify subtypes of asthma. Clin Exp Allergy. 2015; 45:1779–1789.36. Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014; 133:98–103.e1-e6.37. Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013; 110:16987–16992.38. Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012; 119:3790–3798.39. Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014; 133:1692–1701.e3.40. Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-gamma in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013; 132:856–865.e1-e3.41. Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014; 193:41–47.42. Lundström SL, Yang J, Källberg HJ, Thunberg S, Gafvelin G, Haeggström JZ, et al. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One. 2012; 7:e33780.43. Mastalerz L, Januszek R, Kaszuba M, Wójcik K, Celejewska-Wójcik N, Gielicz A, et al. Aspirin provocation increases 8-iso-PGE2 in exhaled breath condensate of aspirin-hypersensitive asthmatics. Prostaglandins Other Lipid Mediat. 2015; 121:163–169.44. Longmire AW, Roberts LJ, Morrow JD. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/endoperoxide receptor in humans and rats: additional evidence for the existence of a unique isoprostane receptor. Prostaglandins. 1994; 48:247–256.45. Machado-Carvalho L, Martin M, Torres R, Gabasa M, AlobidI , Mullol J, et al. Low E-prostanoid 2 receptor levels and deficient induction of the IL-1beta/IL-1 type I receptor/COX-2 pathway: vicious circle in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016; 137:99–107.e7.46. Brugha RE, Mushtaq N, Round T, Gadhvi DH, Dundas I, Gaillard E, et al. Carbon in airway macrophages from children with asthma. Thorax. 2014; 69:654–659.47. Park do Y, Kim CH, Yoon JH, Kim HJ. Alternative method for primary nasal epithelial cell culture using intranasal brushing and feasibility for the study of epithelial functions in allergic rhinitis. Allergy Asthma Immunol Res. 2016; 8:69–78.48. Lundström SL, Balgoma D, Wheelock AM, Haeggström JZ, Dahlén SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Curr Pharm Biotechnol. 2011; 12:1026–1052.49. Rufo JC, Madureira J, Fernandes EO, Moreira A. Volatile organic compounds in asthma diagnosis: a systematic review and meta-analysis. Allergy. 2016; 71:175–188.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Cell-Mediated Autoimmune Responses in Severe Asthma
- Relationship Between Airway Inflammation Assessed by Induced Sputum and Airway Hyperresponsiveness in Asthmatic Patient
- Airway remodelling in asthma: role for mechanical forces
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- Sputum induction method for studying total IgE levels in atopics asthamtic patients