Yonsei Med J.
2015 Nov;56(6):1619-1626. 10.3349/ymj.2015.56.6.1619.
Hsp70 Knockdown by siRNA Decreased Collagen Production in Keloid Fibroblasts
- Affiliations
-
- 1Department of Dermatology, Severance Hospital, Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. juhee@yuhs.ac
- 2Department of Plastic and Reconstructive Surgery, Severance Hospital, Institute for Human Tissue Restoration, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Dermatology and Cutaneous Biology Research Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
- KMID: 2345891
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1619
Abstract
- PURPOSE
There are currently no consistently effective treatments for the excessive collagen produced by keloid fibroblasts. Previously, we reported that heat shock protein 70 (Hsp70) is up-regulated in keloid fibroblasts and keloid tissue. We, therefore, investigated whether Hsp70 is related to excessive collagen production in keloid fibroblasts.
MATERIALS AND METHODS
We inhibited Hsp70 in keloid fibroblasts by RNA interference and examined the resulting collagen expression. Thus, we selected small interfering RNAs (siRNAs) specific for human Hsp70, transfected them into keloid fibroblasts, and evaluated the resulting phenotypes and protein production using real-time polymerase chain reaction (PCR), Western blot, and a collagen assay.
RESULTS
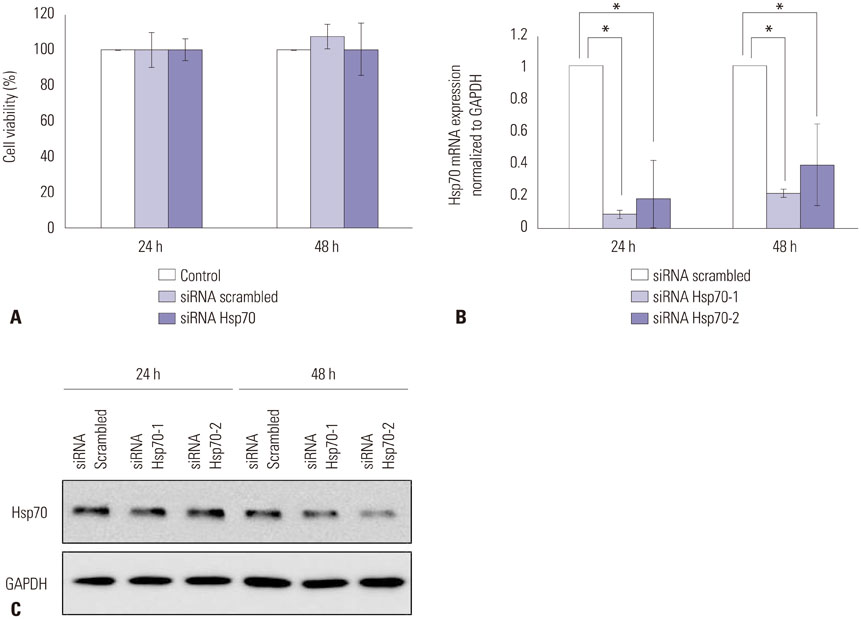
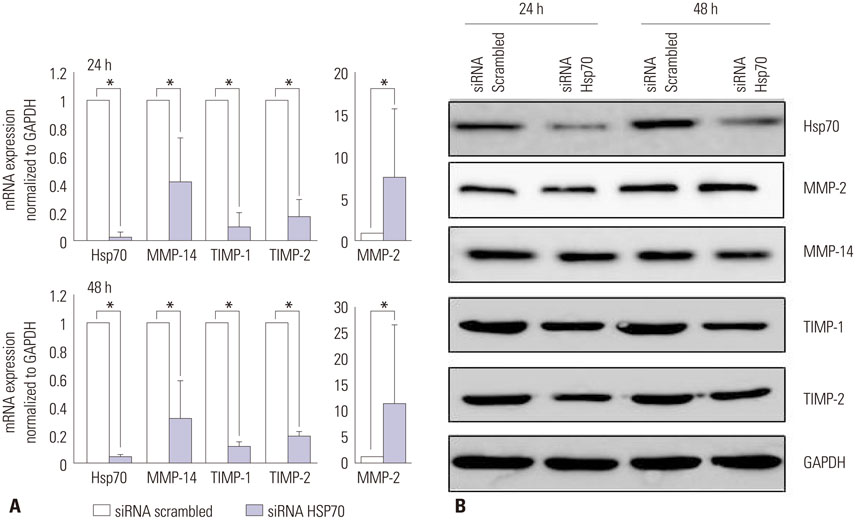
The siRNAs dramatically suppressed Hsp70 mRNA expression, resulting in a decrease in collagen production in the keloid fibroblasts compared with controls. The siRNAs did not influence the viability of the keloid fibroblasts.
CONCLUSION
Hsp70 overexpression likely plays an important role in the excessive collagen production by keloid fibroblasts. RNA interference has therapeutic potential for the treatment of keloids.
Keyword
MeSH Terms
-
Adolescent
Adult
Blotting, Western
Collagen/*drug effects/metabolism
Female
Fibroblasts/metabolism
Gene Expression Regulation
HSP70 Heat-Shock Proteins/genetics/metabolism/*pharmacology
Humans
Keloid/*drug therapy/genetics/metabolism
Male
RNA, Messenger/*genetics
RNA, Small Interfering/*genetics
Real-Time Polymerase Chain Reaction
Transfection
Up-Regulation
Collagen
HSP70 Heat-Shock Proteins
RNA, Messenger
RNA, Small Interfering
Figure
Reference
-
1. Mukhopadhyay A, Tan EK, Khoo YT, Chan SY, Lim IJ, Phan TT. Conditioned medium from keloid keratinocyte/keloid fibroblast coculture induces contraction of fibroblast-populated collagen lattices. Br J Dermatol. 2005; 152:639–645.2. Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998; 4:19–24.
Article3. Poochareon VN, Berman B. New therapies for the management of keloids. J Craniofac Surg. 2003; 14:654–657.
Article4. Chen W, Fu X, Sun X, Sun T, Zhao Z, Sheng Z. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003; 113:208–216.
Article5. Chen W, Fu XB, Ge SL, Sun XQ, Zhou G, Zhao ZL, et al. Development of gene microarray in screening differently expressed genes in keloid and normal-control skin. Chin Med J (Engl). 2004; 117:877–881.6. Chen JJ, Zhao S, Cen Y, Liu XX, Yu R, Wu DM. Effect of heat shock protein 47 on collagen accumulation in keloid fibroblast cells. Br J Dermatol. 2007; 156:1188–1195.
Article7. Lee JH, Shin JU, Jung I, Lee H, Rah DK, Jung JY, et al. Proteomic profiling reveals upregulated protein expression of hsp70 in keloids. Biomed Res Int. 2013; 2013:621538.
Article8. Totan S, Echo A, Yuksel E. Heat shock proteins modulate keloid formation. Eplasty. 2011; 11:e21.9. Javad F, Day PJ. Protein profiling of keloidal scar tissue. Arch Dermatol Res. 2012; 304:533–540.
Article10. Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996; 1:23–28.
Article11. Zhang GY, Yi CG, Li X, Ma B, Li ZJ, Chen XL, et al. Troglitazone suppresses transforming growth factor-beta1-induced collagen type I expression in keloid fibroblasts. Br J Dermatol. 2009; 160:762–770.
Article12. Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992; 355:33–45.
Article13. Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996; 111:345–357.
Article14. Mathew A, Morimoto RI. Role of the heat-shock response in the life and death of proteins. Ann N Y Acad Sci. 1998; 851:99–111.
Article15. Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010; 40:253–266.
Article16. Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007; 179:1236–1244.
Article17. Naitoh M, Hosokawa N, Kubota H, Tanaka T, Shirane H, Sawada M, et al. Upregulation of HSP47 and collagen type III in the dermal fibrotic disease, keloid. Biochem Biophys Res Commun. 2001; 280:1316–1322.
Article18. Uitto J, Perejda AJ, Abergel RP, Chu ML, Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985; 82:5935–5939.
Article19. Ala-Kokko L, Rintala A, Savolainen ER. Collagen gene expression in keloids: analysis of collagen metabolism and type I, III, IV, and V procollagen mRNAs in keloid tissue and keloid fibroblast cultures. J Invest Dermatol. 1987; 89:238–244.
Article20. Peltonen J, Hsiao LL, Jaakkola S, Sollberg S, Aumailley M, Timpl R, et al. Activation of collagen gene expression in keloids: co-localization of type I and VI collagen and transforming growth factor-beta 1 mRNA. J Invest Dermatol. 1991; 97:240–248.
Article21. Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011; 164:83–96.
Article22. Gao Z, Wang Z, Shi Y, Lin Z, Jiang H, Hou T, et al. Modulation of collagen synthesis in keloid fibroblasts by silencing Smad2 with siRNA. Plast Reconstr Surg. 2006; 118:1328–1337.
Article23. Asano T, Tanaka K, Yamakawa N, Adachi H, Sobue G, Goto H, et al. HSP70 confers protection against indomethacin-induced lesions of the small intestine. J Pharmacol Exp Ther. 2009; 330:458–467.
Article24. Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, et al. Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011; 31:5225–5234.
Article25. Matsuda M, Hoshino T, Yamashita Y, Tanaka K, Maji D, Sato K, et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J Biol Chem. 2010; 285:5848–5858.
Article26. Suemasu S, Tanaka K, Namba T, Ishihara T, Katsu T, Fujimoto M, et al. A role for HSP70 in protecting against indomethacin-induced gastric lesions. J Biol Chem. 2009; 284:19705–19715.
Article27. Tanaka K, Mizushima T. Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperthermia. 2009; 25:668–676.
Article28. Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, et al. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007; 282:23240–23252.
Article29. Tanaka K, Tsutsumi S, Arai Y, Hoshino T, Suzuki K, Takaki E, et al. Genetic evidence for a protective role of heat shock factor 1 against irritant-induced gastric lesions. Mol Pharmacol. 2007; 71:985–993.
Article30. Tanaka K, Tanaka Y, Namba T, Azuma A, Mizushima T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2010; 80:920–931.
Article31. Namba T, Tanaka K, Hoshino T, Azuma A, Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PLoS One. 2011; 6:e27296.
Article32. Chen JJ, Jin PS, Zhao S, Cen Y, Liu Y, Xu XW, et al. Effect of heat shock protein 47 on collagen synthesis of keloid in vivo. ANZ J Surg. 2011; 81:425–430.
Article33. Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007; 15:Suppl 1. S46–S53.
Article34. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005; 153:295–300.
Article35. Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen. 1999; 7:166–171.
Article36. Imaizumi R, Akasaka Y, Inomata N, Okada E, Ito K, Ishikawa Y, et al. Promoted activation of matrix metalloproteinase (MMP)-2 in keloid fibroblasts and increased expression of MMP-2 in collagen bundle regions: implications for mechanisms of keloid progression. Histopathology. 2009; 54:722–730.
Article37. Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, et al. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J Biol Chem. 1998; 273:1216–1222.
Article38. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998; 273:871–880.
Article39. Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006; 6:1215–1225.
Article40. Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000; 87:12–19.
Article41. Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000; 191:245–256.
Article42. Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci U S A. 1997; 94:7959–7964.
Article43. Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995; 55:3263–3266.44. Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003; 22:55–61.
Article45. Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol. 2003; 54:1–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidermal Growth Factor (EGF)-Like Repeats and Discoidin I-Like Domains 3 (EDIL3): A Potential New Therapeutic Tool for the Treatment of Keloid Scars
- Influence of Keloid-derived Keratinocyte on TGF- beta1 Production of Fibroblast in Co-culture Model
- The Effect of Tumor Necrosis Factor-alpa on Type I Procollagen and Collagenase Gene Expression in Hypertrophic Scar and Keloid Fibroblast
- Anti-fibrotic Effect of Silibinin in Keloid Fibroblasts and a Bleomycin-induced, Scleroderma-like Animal Model
- Effects of Estrogens and Isoflavones on the Collagen Synthesis