J Korean Soc Spine Surg.
2001 Dec;8(4):447-454.
Matrix Synthesis of Human Intervertebral Disc Cells: Effect of Gene Transfer, Exogenous Growth Factor, Incubation Period, and Culture Methods
- Affiliations
-
- 1Department of Orthopedic Surgery, Yonsei University College of Medicine, Seoul, Korea. shmoon@yumc.yonsei.ac.kr
- 2Department of Orthopaedic Surgery, University of Pittsburgh, USA.
Abstract
-
STUDY DESIGN: In vitro experiment to determine the matrix synthesis of intervertebral disc (IVD) cell to various biologic interventions and conditions.
OBJECTIVES
To elucidate biologic responses in terms of matrix synthesis of human IVD cells in vitro to various factors i.e. concentration of adenoviral vector and exogenous growth factor, duration of incubation, and type of culture methods. SUMMARY OF LITERATURE REVIEW: Sophisticated method to delivery of growth factors, in continuous manner, is the genetic modification of disc cells through gene transfer. Direct comparison of gene transfer and exogenous growth factor on matrix synthesis has not been reported.
MATERIALS AND METHODS
IVD tissue was obtained from twenty three patients. Isolation and preparation of disc cells in monolayer (D) and alginate beads (3D) culture were performed. Disc cells in 2D and 3D were treated with either Ad/TGF-beta1 or exogenous TGF-beta1. Control cultures were treated with either saline or Ad/luciferase. Matrix synthesis (newly synthesized proteoglycan) was measured in various conditions (concentration of adenoviral vector and exogenous growth factor, duration of incubation, and type of culture methods). Newly synthesized proteoglycan were analyzed using chromatography on Sephadex G-25 in PD-10 columns after S35-sulfate incorporation.
RESULTS
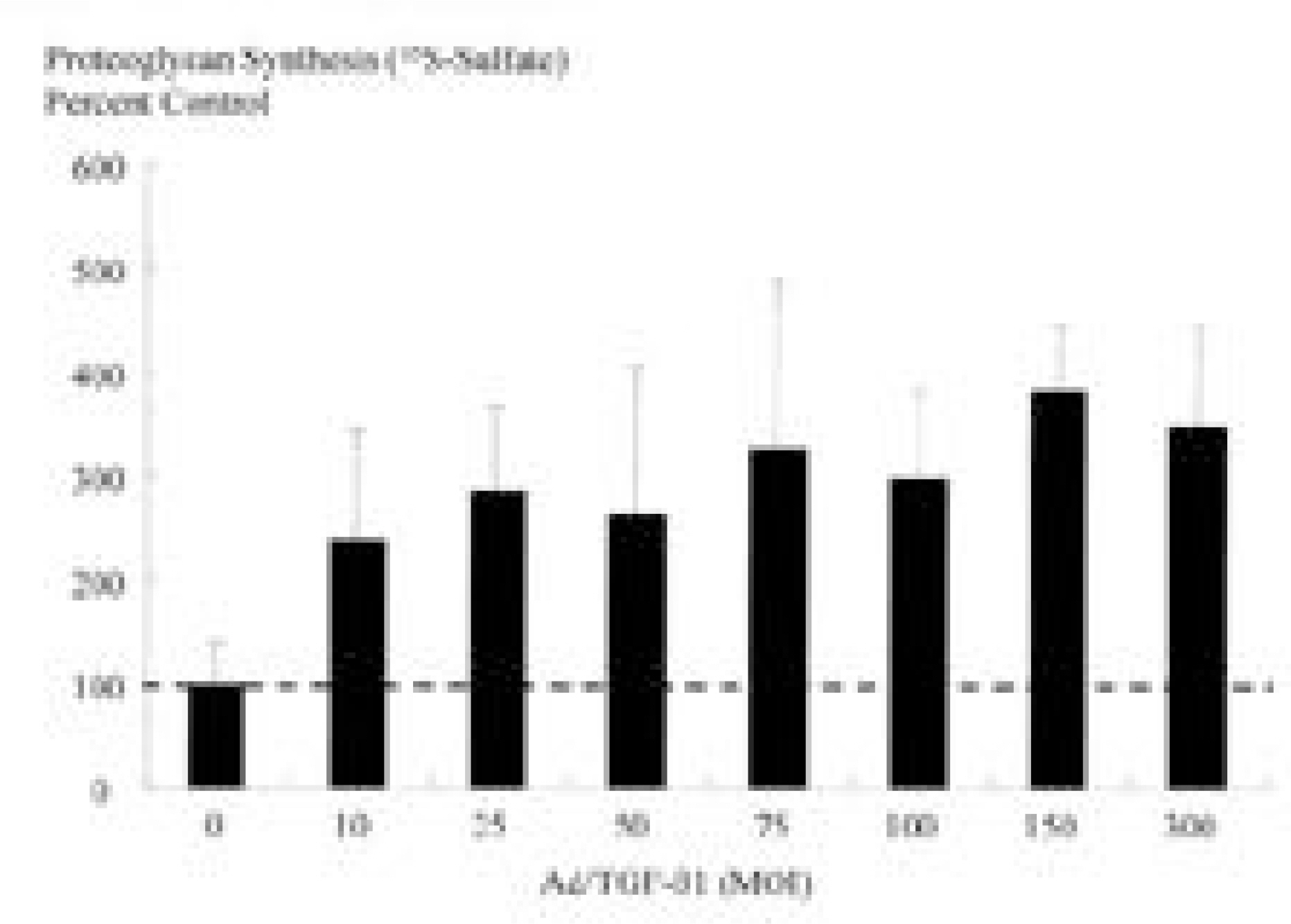
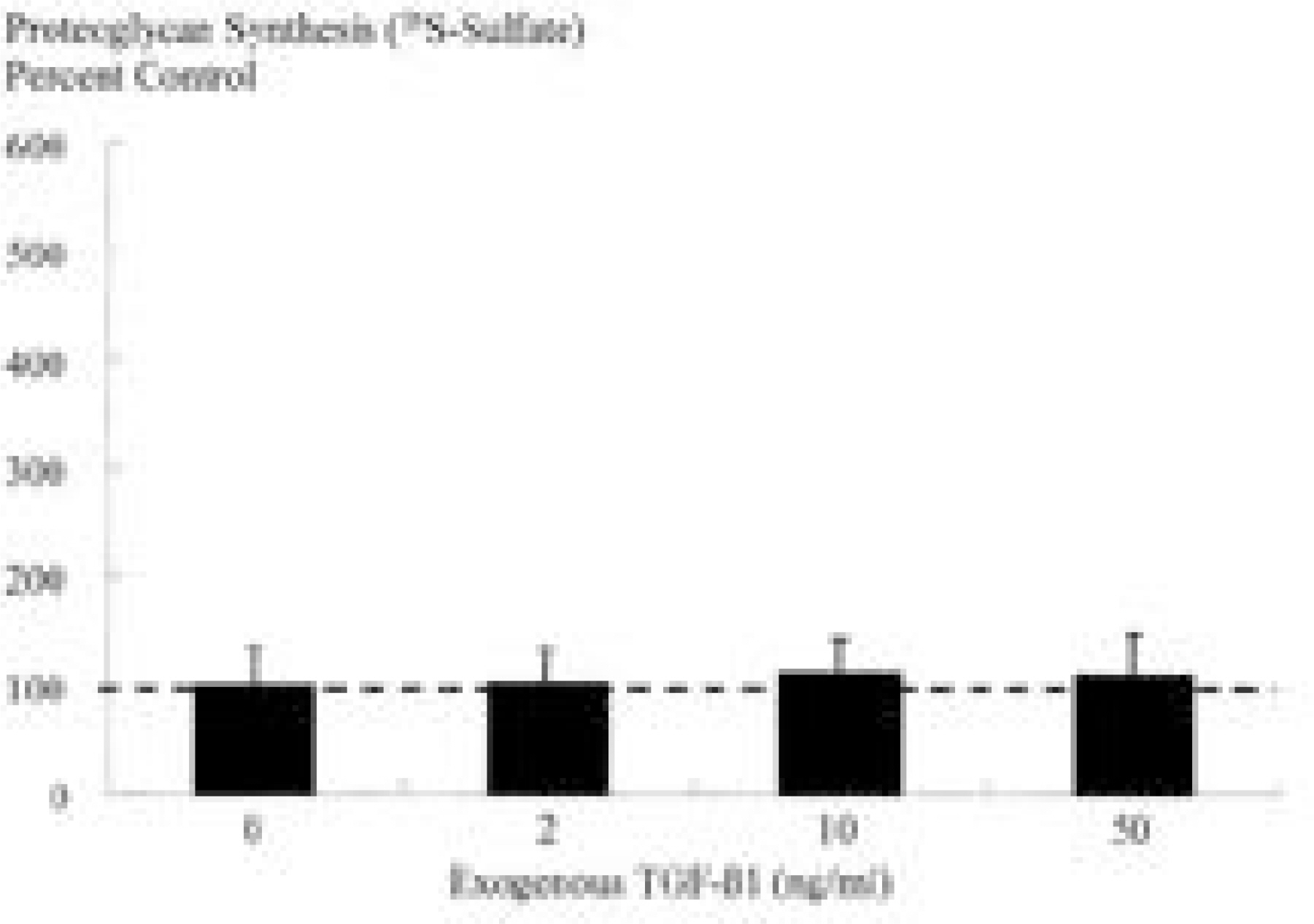
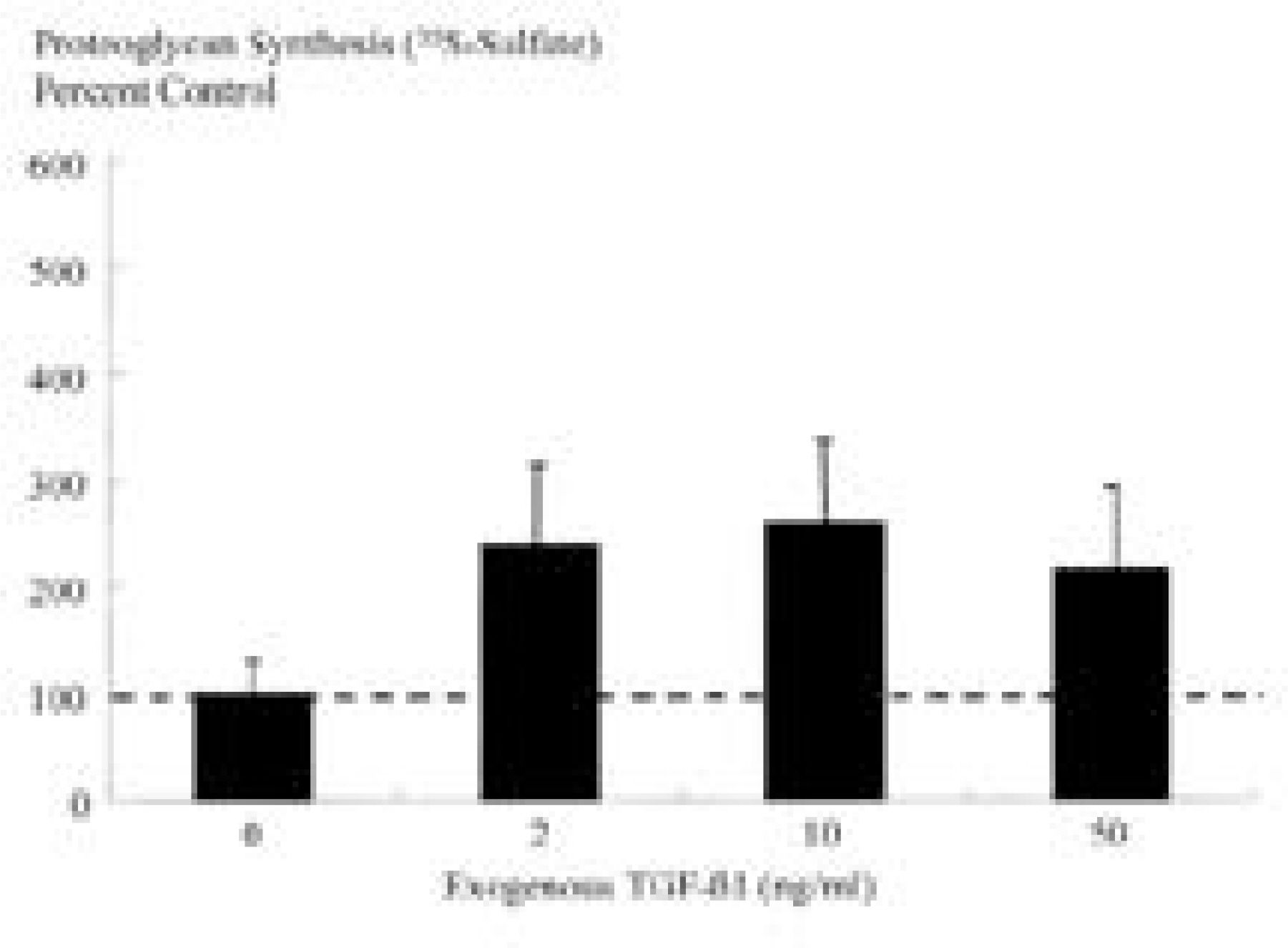
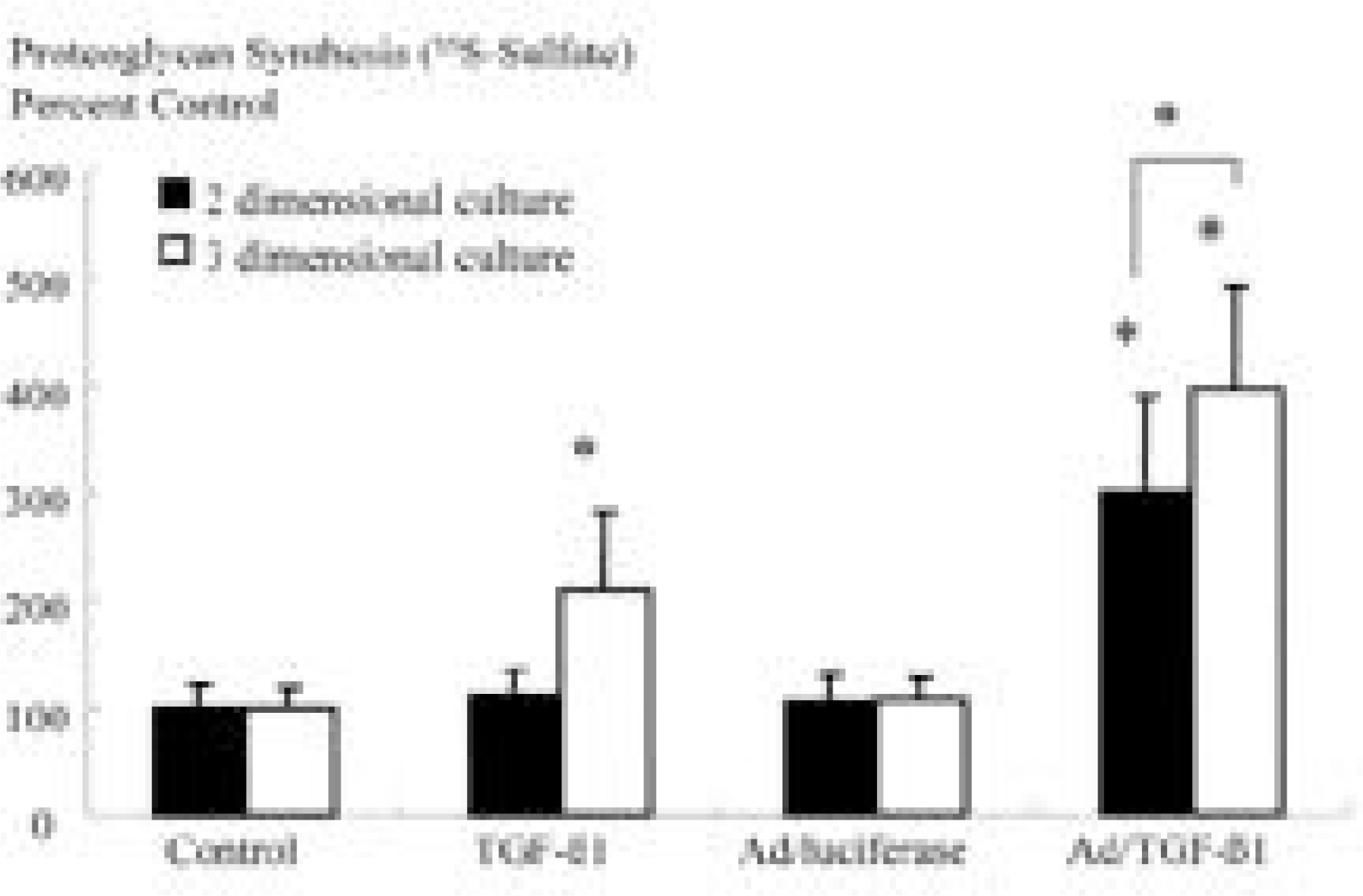
Ad/TGF-beta1 showed increase in proteoglycan synthesis (plateau at 75MOI) in 3D culture, (plateau at 25MOI) in 2D culture. In 3D culture, Ad/TGF-beta1 showed significant increase in proteoglycan synthesis on day 1, 2, and 3 of incubation. In 2D culture, Ad/TGF-beta1 showed significant increase in proteoglycan synthesis on day 2 of incubation with significant loss of anabolic effect on day 3. In 3D culture, exogenous TGF-beta1 showed increase in proteoglycan synthesis (plateau at 2ng/ml) while in 2D culture, there is no synthetic response to exogenous TGF-beta1
CONCLUSION
Therapeutic gene transfer provided sustained and increased anabolic responses than exogenous growth factor.
MeSH Terms
Figure
Reference
-
1). Anderson JAD. Back pain and occupation. The lumbar Spine and Back Pain. Third Edition, Edited by MIV Jayson. London. Churchill Livingstone;1987. PP2-36.2). Aydelotte MB, Greenhill RR, Kuettner KE. Differ -ences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tiss Res. 18:223–234. 1988.3). Boden SD, Schimandle JH, Hutton WC. 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part II: Study of dose, carrier, and species. Spine. 20:2633–2644. 1995.4). Boden SD, Schimandle JH, Hutton WC, et al. 199 5 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine. 20:2626–2632. 1995.5). Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 4:226–232. 1992.
Article6). Brown GL, Curtsinger LJ, White M, et al. Acceleration of tensile strength of incisions treated with EGF and TGF-. Ann. Surg. 208:788–794. 1988.7). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 20:1307–1314. 1995.
Article8). Butler D, Trafimow JH, Andersson GB, et al. Discs degenerate before facets. Spine. 15:111–113. 1990.
Article9). Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogenous cell populations in normal human intervertebral disc. J Anat. 186:43–53. 1995.10). Chiba K, Andersson GBJ, Masuda K, et al. Metaboli sm of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine. 22:2885–2893. 1997.11). Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg(Am). 77:1103–1113. 1995.
Article12). Eyre D, Benya P, Buckwalter J, et al. Intervertebral disc: Part B. Basic science perspective. New Perspectives in Low Back Pain. Park Ridge, IL: American Academy of Orthopaedic Surgeons;p. 147–207. 1989.13). Graham FL and Eb Ajvd. A new technique for assay of infectivity of human adenovirus 5 DNA. Virol. 52:456–467. 1973.14). Guo J, Jourdian GW, McCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Conn Tiss Res. 19:277–97. 1989.
Article15). Gruber HE, Fisher EC, Desani B, et al. Human intervertebral disc cells from the annulus: three dimensional culture in agarose or alginate and responsiveness to TGF-b1. Exp Cell Res. 235:13–21. 1997.16). Hauselmann HJ, Fernandes RJ, Mok SS, et al. Phe no -typic stability of bovine articular chondrocytes after longterm culture in alginate beads. J Cell Sci. 107:17–27. 1994.17). Lipson SJ, Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 6:194–210. 1981.
Article18). Maldonado BA, Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 10:677–690. 1992.
Article19). Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 70:7498–7509. 1996.
Article20). Moon S-H, Kang JD, Nishida K, et al. Human cervical intervertebral disc cells are susceptible to adenovirus-mediated gene therapy. Proceedings of Cervical Spine Research Society. 1999.21). Moon S-H, Nishida K, Kang JD, et al. Human intervertebral disc cells are genetically modifiable in-vitro by ade -novirus-mediated gene transfer: Implications for the treatment of degenerative disc disease. Proceedings of North American Spine Society. 1999.22). Nishida K, Kang JD, Suh J-K, et al. Adenovirus -mediat -ed gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 23:2437–2443. 1998.23). Nishida K, Kang JD, Gilbertson LG, et al. Mod ulation of biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factors 1 encoding gene. Spine. 24:2419–2425. 1999.24). Osada R, Oshima H, Ishihara H, et al. Autocrine/parac-rine mechanism of insulin-like growth factor-1 secretion, and the effect of insuline-like growth factors-1 on proteoglycan synthesis in bovine intervertebral discs. J Orhtop Res. 14:690–699. 1996.25). Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 80:35–47. 1998.
Article26). Siegel JA, Lonner BS, Grande DA, James T. The effect of transforming growth factor-beta on intervertebral disc tissue. Proceedings of ISSLS, 213A, Kona Hawaii. 1999.27). Sprugel KH, McPherson JM, Clowes AW, et al. Effect of growth factors in vivo. Am J Pathol. 129:601–613. 1987.28). Takemi K, Kumano F, An H, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annu -lus fibrosus cells to repair their matrix after chondroitinase ABC-induced chemonucleolysis. Tranc Orthop Res Soc. 201:1999.29). Thompson JP, Oegema TR Jr, Bradford DS. Stimulation of mature cannine intervertebral disc by growth factors. Spine. 16:253–260. 1991.30). Wakefield LM, Winokur TS, Hollands RS, et al. Re-combinant latent transforming growth factor ß 1 has a longer plasma half-life in rats than active transforming growth factor ß 1, and a different tissue distribution. J Clin Invest. 86:1976–1984. 1990.31). Yeh P, Perricaudet M. Advances in adenoviral vectors: from genetic engineering to their biology. FASEB Journal. 11:615–623. 1997.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenovirus-Mediated Therapeutic Gene Transfer: Matrix Synthesis of Human Intervertebral Disc Cells
- Biological Effect of TGF - B 1 on Human Intervertebral Disc by Cell Culture System
- Responsiveness of Human Intervertebral Disc Cells in Matrix Synthesis To Adenovirus-Mediated Gene Transfer(IGF-1, TGF-beta1 Encoding Genes)
- Matrix Synthesis of Human Intervertebral Disc Cells According to Grade of Degeneration: Under the Basal State and TGF-beta Stimulation
- Transforming Growth Factor-beta1 and Bone Morphogenetic Protein-2 Upregulates Matrix Synthesis and Chondrogenic Phenotype in Intervertebral Disc Cells