Tuberc Respir Dis.
2014 Mar;76(3):114-119.
Schedule-Dependent Effect of Epigallocatechin-3-Gallate (EGCG) with Paclitaxel on H460 Cells
- Affiliations
-
- 1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. chestor@hallym.or.kr
Abstract
- BACKGROUND
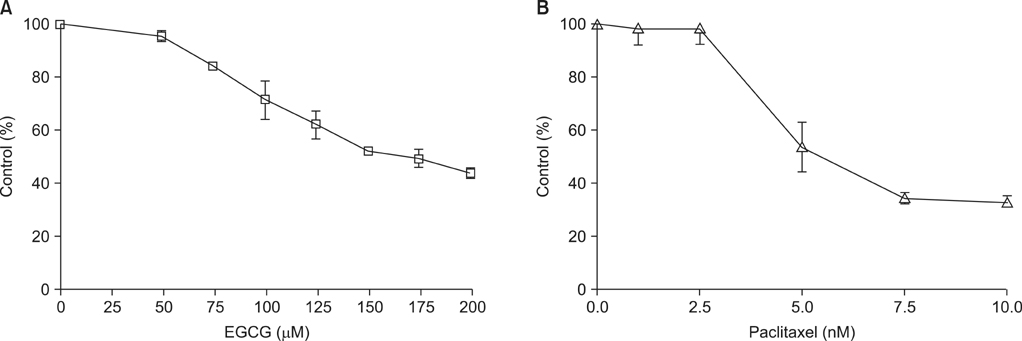
Epigallocatechin-3-gallate (EGCG), a major biologically active component of green tea, has anti-cancer activity in human and animal models. We investigated the schedule-dependent effect of EGCG and paclitaxel on growth of NCI-H460 non-small cell lung cancer cells.
METHODS
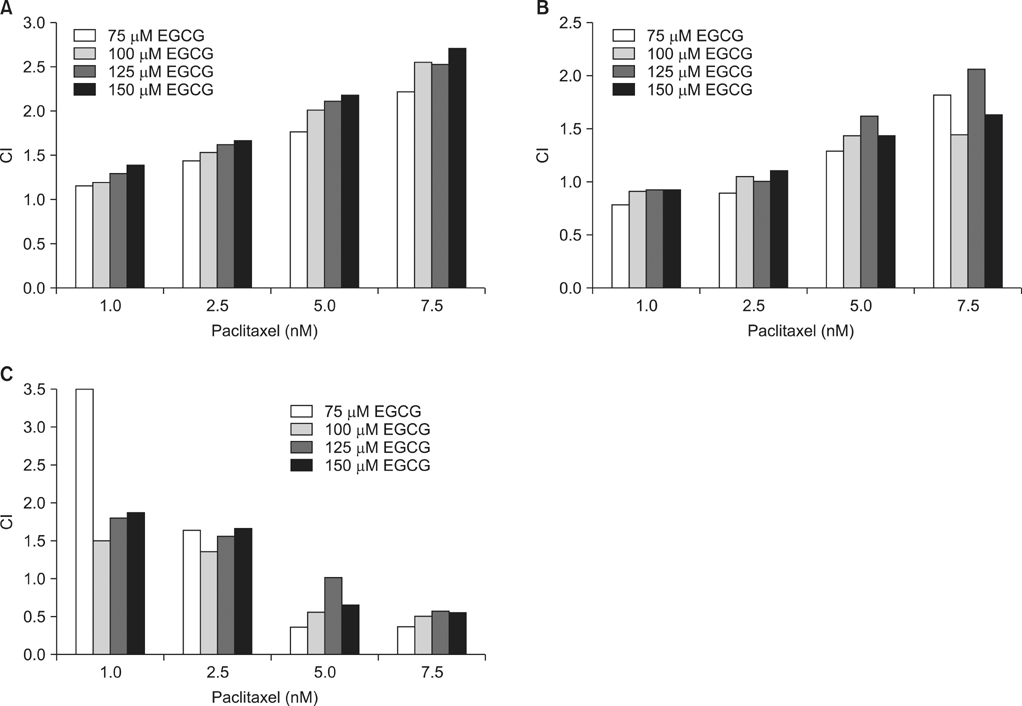
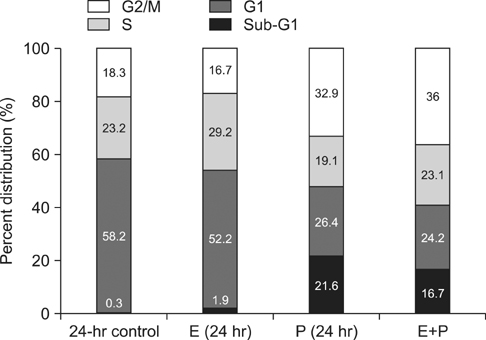
To investigate the combined effect of EGCG (E) and paclitaxel (P), combination indices (CIs) were calculated, and cell cycle analysis was performed. For the effect on cell apoptosis, western blot analysis was also performed.
RESULTS
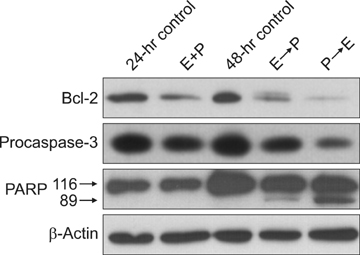
CI analysis demonstrated that both concurrent and sequential E --> P treatments had antagonistic effects (CIs >1.0), but sequential P --> E had synergistic effects (CIs <1.0), on the growth inhibition of NCI-H460 cells. In the cell cycle analysis, although paclitaxel induced G2/M cell cycle arrest and increased the sub-G1 fraction, concurrent EGCG and paclitaxel treatments did not have any additive or synergistic effects compared with the paclitaxel treatment alone. However, western blot analysis demonstrated that sequential P --> E treatment decreased the expression of Bcl-2 and procaspase-3 and increased poly(ADP-ribose) polymerase (PARP) cleavage; while minimal effects were seen with concurrent or sequential E --> P treatments.
CONCLUSION
Concurrent or sequential E --> P treatment had opposite effects to P --> E treatment, where P --> E treatment showed a synergistic effect on growth inhibition of NCI-H460 cells by inducing apoptosis. Thus, the efficacy of EGCG and paclitaxel combination treatment seems to be schedule-dependent.
MeSH Terms
Figure
Reference
-
1. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997; 26:769–775.2. Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997; 70:255–258.3. Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res. 1990; 81:1101–1108.4. Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF Jr. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994; 86:855–858.5. Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997; 387:561.6. Komori A, Yatsunami J, Okabe S, Abe S, Hara K, Suganuma M, et al. Anticarcinogenic activity of green tea polyphenols. Jpn J Clin Oncol. 1993; 23:186–190.7. Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993; 85:1038–1049.8. Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150:43–56.9. Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003; 133:3262S–3267S.10. Laurie SA, Miller VA, Grant SC, Kris MG, Ng KK. Phase I study of green tea extract in patients with advanced lung cancer. Cancer Chemother Pharmacol. 2005; 55:33–38.11. Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review). Int J Cancer. 1999; 83:151–156.12. Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999; 17:1061–1070.13. Cheng H, An SJ, Zhang XC, Dong S, Zhang YF, Chen ZH, et al. In vitro sequence-dependent synergism between paclitaxel and gefitinib in human lung cancer cell lines. Cancer Chemother Pharmacol. 2011; 67:637–646.14. Oliveras-Ferraros C, Vazquez-Martin A, Colomer R, De Llorens R, Brunet J, Menendez JA. Sequence-dependent synergism and antagonism between paclitaxel and gemcitabine in breast cancer cells: the importance of scheduling. Int J Oncol. 2008; 32:113–120.15. Zoli W, Ricotti L, Barzanti F, Dal Susino M, Frassineti GL, Milandri C, et al. Schedule-dependent interaction of doxorubicin, paclitaxel and gemcitabine in human breast cancer cell lines. Int J Cancer. 1999; 80:413–416.16. Kano Y, Sakamoto S, Kasahara T, Akutsu M, Inoue Y, Miura Y. Effects of amsacrine in combination with other anticancer agents in human acute lymphoblastic leukemia cells in culture. Leuk Res. 1991; 15:1059–1066.17. Zhao L, Wientjes MG, Au JL. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004; 10:7994–8004.18. Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005; 114:513–521.19. Inaba H, Nagaoka Y, Kushima Y, Kumagai A, Matsumoto Y, Sakaguchi M, et al. Comparative examination of anti-proliferative activities of (-)-epigallocatechin gallate and (--)-epigallocatechin against HCT116 colorectal carcinoma cells. Biol Pharm Bull. 2008; 31:79–84.20. Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007; 360:233–237.21. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43:89–143.22. Huh SW, Bae SM, Kim YW, Lee JM, Namkoong SE, Lee IP, et al. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol Oncol. 2004; 94:760–768.23. Shimoyama T, Koizumi F, Fukumoto H, Kiura K, Tanimoto M, Saijo N, et al. Effects of different combinations of gefitinib and irinotecan in lung cancer cell lines expressing wild or deletional EGFR. Lung Cancer. 2006; 53:13–21.24. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011; 82:1807–1821.25. Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, et al. Synergistic inhibition of head and neck tumor growth by green tea (-)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008; 123:1005–1014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversal of Cisplatin Resistance by Epigallocatechin Gallate Is Mediated by Downregulation of Axl and Tyro 3 Expression in Human Lung Cancer Cells
- Epigallocatechin-3-gallate Regulates Inducible Nitric Oxide Synthase Expression in Human Umbilical Vein Endothelial Cells
- Induction of Apoptosis by (-)-epigallocatechin-3-gallate in HL-60 Cells
- Protective Effects of Epigallocatechin Gallate After UV Irradiation of Cultured Human Lens Epithelial Cells
- Protective Effects of Epigallocatechin Gallate after UV Irradiation in Cultured Human Retinal Pigment Epithelial Cells