Tuberc Respir Dis.
2014 Jan;76(1):15-22.
The Significance of Caspase-Cleaved Cytokeratin 18 in Pleural Effusion
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Korea. parkkj@ajou.ac.kr
- 2Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
Abstract
- BACKGROUND
Apoptosis plays a role in the development of pleural effusion. Caspase-cleaved cytokeratin 18, a marker for epithelial cell apoptosis, was evaluated in pleural effusion.
METHODS
A total of 79 patients with pleural effusion were enrolled. The underlying causes were lung cancer (n=24), parapneumonic effusion (n=15), tuberculous effusion (n=28), and transudates (n=12). The levels of M30, an epitope of caspase-cleaved cytokeratin 18, were measured in blood and pleural fluids using enzyme-linked immunosorbent assay along with routine cellular and biochemical parameters. The expression of M30 was evaluated in the pleural tissues using immunohistochemistry for M30.
RESULTS
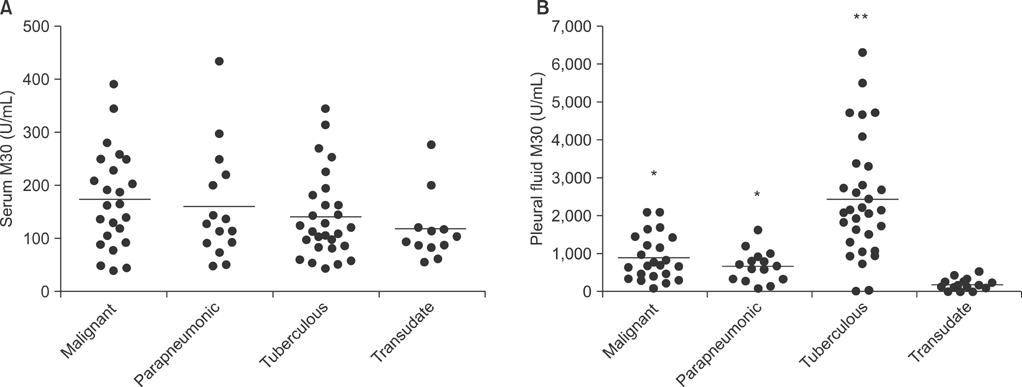
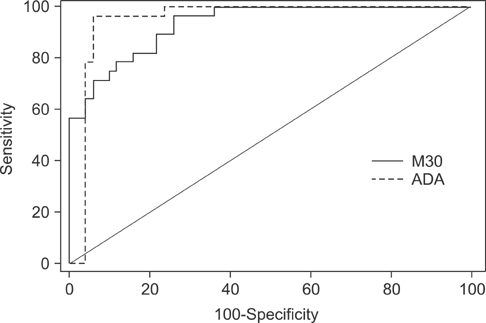
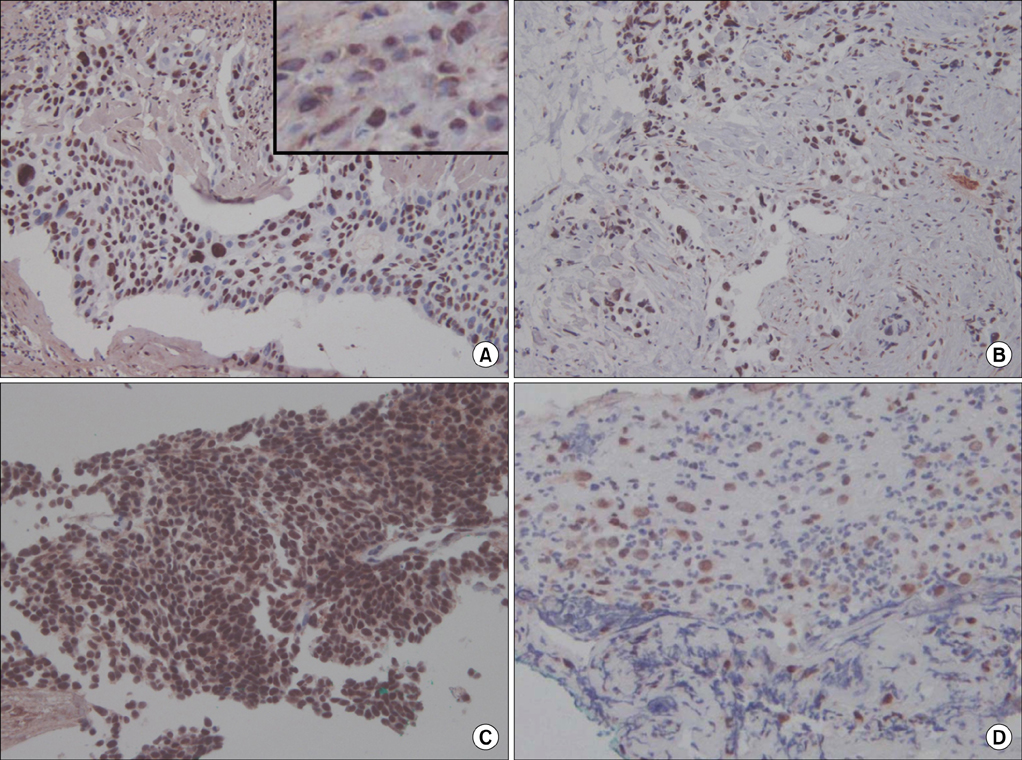
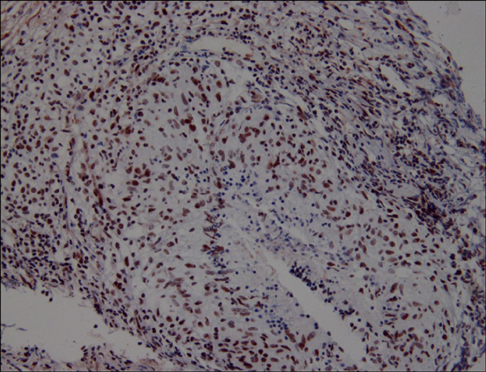
The M30 levels in pleural fluid were significantly higher in patients with tuberculosis (2,632.1+/-1,467.3 U/mL) than in patients with lung cancer (956.5+/-618.5 U/mL), parapneumonic effusion (689.9+/-413.6 U/mL), and transudates (273.6+/-144.5 U/mL; all p<0.01). The serum levels were not significantly different among the disease groups. Based on receiver operating characteristics analysis, the area under the curve of M30 for differentiating tuberculous pleural effusion from all other effusions was 0.93. In the immunohistochemical analysis of M30, all pathologic types of cancer cells showed moderate to high expression, and the epithelioid cells in granulomas showed high expression in tuberculous pleural tissues.
CONCLUSION
Caspase-cleaved cytokeratin 18 was most prominently observed in tuberculous pleural effusion and showed utility as a clinical marker. The main source of M30 was found to be the epithelioid cells of granulomas in tuberculous pleural tissues.
MeSH Terms
Figure
Reference
-
1. van den Berg E, van Woensel JB, Bem RA. Apoptosis in pneumovirus infection. Viruses. 2013; 5:406–422.2. Zielinski RR, Eigl BJ, Chi KN. Targeting the apoptosis pathway in prostate cancer. Cancer J. 2013; 19:79–89.3. Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002; 9:367–393.4. Barrett KL, Willingham JM, Garvin AJ, Willingham MC. Advances in cytochemical methods for detection of apoptosis. J Histochem Cytochem. 2001; 49:821–832.5. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010; 277:301–308.6. Yuan Y, Liao YM, Hsueh CT, Mirshahidi HR. Novel targeted therapeutics: inhibitors of MDM2, ALK and PARP. J Hematol Oncol. 2011; 4:16.7. Wu SH, Li CT, Lin CH, Chu JJ, Cheng ML, Lin KH. Soluble Fas ligand is another good diagnostic marker for tuberculous pleurisy. Diagn Microbiol Infect Dis. 2010; 68:395–400.8. de Matos LL, Del Giglio AB, Matsubayashi CO, de Lima Farah M, Del Giglio A, da Silva Pinhal MA. Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: systematic review and diagnostic meta-analysis. Diagn Pathol. 2012; 7:97.9. Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013; 108:1526–1531.10. Kawai M, Saegusa Y, Kemmochi S, Harada T, Shimamoto K, Shibutani M, et al. Cytokeratin 8/18 is a useful immunohistochemical marker for hepatocellular proliferative lesions in mice. J Vet Med Sci. 2010; 72:263–269.11. De Petris L, Branden E, Herrmann R, Sanchez BC, Koyi H, Linderholm B, et al. Diagnostic and prognostic role of plasma levels of two forms of cytokeratin 18 in patients with non-small-cell lung cancer. Eur J Cancer. 2011; 47:131–137.12. Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004; 214:1–9.13. Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005; 59:Suppl 2. S359–S362.14. Chung WY, Sun JS, Park JH, Lee HL, Lee KS, Kim YS, et al. Epithelial apoptosis as a clinical marker in idiopathic interstitial pneumonia. Respir Med. 2010; 104:1722–1728.15. Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999; 187:567–572.16. Roth GA, Krenn C, Brunner M, Moser B, Ploder M, Spittler A, et al. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope m30 in critically ill patients. Shock. 2004; 22:218–220.17. Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009; 175:808–816.18. Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007; 56:399–404.19. Lee KS, Choi YH, Kim YS, Baik SH, Oh YJ, Sheen SS, et al. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med. 2008; 102:464–469.20. Sikora J, Dworacki G, Zeromski J. Expression of Fas and Fas ligand and apoptosis in tumor-associated lymphocytes and in tumor cells from malignant pleural effusions. Nat Immun. 1998; 16:244–255.21. Wang PS, Chen YM, Hsieh YL, Yu CF, Tsai CM, Perng RP. Pleural effusion and serum soluble fas-ligand levels are elevated in different clinical conditions. Lung. 2002; 180:25–32.22. Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980; 69:507–512.23. Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri P, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013; 17:e76–e83.24. Zysk G, Bejo L, Schneider-Wald BK, Nau R, Heinz H. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin Exp Immunol. 2000; 122:61–66.25. Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006; 74:6135–6144.26. Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003; 5:649–660.27. Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997; 65:298–304.28. Kahn HJ, Hanna W, Yeger H, Baumal R. Immunohistochemical localization of prekeratin filaments in benign and malignant cells in effusions: comparison with intermediate filament distribution by electron microscopy. Am J Pathol. 1982; 109:206–214.29. van Engeland M, Kuijpers HJ, Ramaekers FC, Reutelingsperger CP, Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp Cell Res. 1997; 235:421–430.30. Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004; 297:11–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Tools of Pleural Effusion
- Clinical Significance of Pleural Effusion in Patients with Aeute Pancreatitis
- The Utility of Pleural Adenosine Deaminase for Diagnosis of Differentiating Tuberculous Pleural Effusion in Children
- Incidence and Significance of Pleural Effusion after Hepatoma Surgery
- Diagnostic Thoracoscopy in the Pleural Effusion