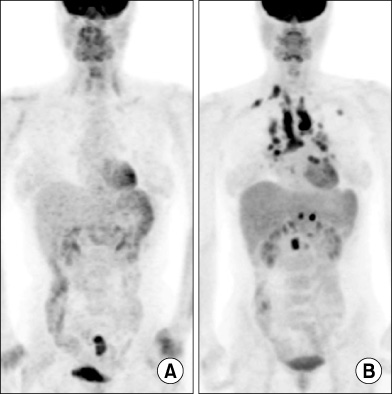
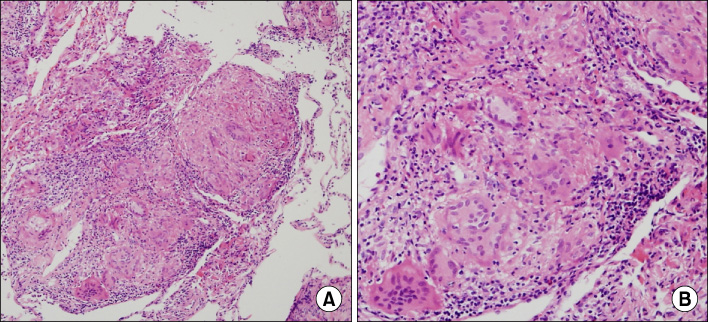
Tuberc Respir Dis.
2012 Mar;72(3):318-322.
A Case of Capecitabine-Induced Sarcoidosis
- Affiliations
-
- 1Center for Lung Cancer, National Cancer Center, Goyang, Korea. jekyde7@gmail.com
- 2Department of Pulmonary and Critical Care Medicine, Gachon University Gil Hospital, Incheon, Korea.
- 3Center for Colorectal Cancer, National Cancer Center, Goyang, Korea.
Abstract
- Sarcoidosis is an inflammatory disease involving multiple-organs with an unknown cause. The new onset of sarcoidosis associated with therapeutic agents has been observed in 3 clinical settings; tumor necrosis factor antagonists in autoimmune rheumatologic diseases, interferon alpha with or without ribavirin in patients with chronic hepatitis C or melanoma, and antineoplastic agent-associated sarcoidosis in patients with hematologic malignancies. Here, we report a female patient who developed sarcoidosis after capecitabine treatment as an adjuvant chemotherapy for sigmoid colon cancer. To our knowledge, this is the first report of a capecitabine-induced sarcoidosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011. 183:573–581.2. Morgenthau AS, Iannuzzi MC. Recent advances in sarcoidosis. Chest. 2011. 139:174–182.3. Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004. 170:1324–1330.4. Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001. 164(10 Pt 1):1885–1889.5. Mikhail SE, Sun JF, Marshall JL. Safety of capecitabine: a review. Expert Opin Drug Saf. 2010. 9:831–841.6. Clementine RR, Lyman J, Zakem J, Mallepalli J, Lindsey S, Quinet R. Tumor necrosis factor-alpha antagonist-induced sarcoidosis. J Clin Rheumatol. 2010. 16:274–279.7. Ramos-Casals M, Perez-Alvarez R, Perez-de-Lis M, Xaubet A, Bosch X. BIOGEAS Study Group. Pulmonary disorders induced by monoclonal antibodies in patients with rheumatologic autoimmune diseases. Am J Med. 2011. 124:386–394.8. Ramos-Casals M, Mañá J, Nardi N, Brito-Zerón P, Xaubet A, Sánchez-Tapias JM, et al. Sarcoidosis in patients with chronic hepatitis C virus infection: analysis of 68 cases. Medicine (Baltimore). 2005. 84:69–80.9. Gayet AR, Plaisance P, Bergmann JF, Mouly S. Development of sarcoidosis following completion of treatment for hepatitis C with pegylated interferon-{alpha2}a and ribavirin: a case report and literature review. Clin Med Res. 2010. 8:163–167.10. Heinzerling LM, Anliker MD, Müller J, Schlaeppi M, von Moos R. Sarcoidosis induced by interferon-alpha in melanoma patients: incidence, clinical manifestations, and management strategies. J Immunother. 2010. 33:834–839.11. López V, Molina I, Monteagudo C, Jordá E. Cutaneous sarcoidosis developing after treatment with pegylated interferon and ribavirin: a new case and review of the literature. Int J Dermatol. 2011. 50:287–291.12. Papanikolaou IC, Sharma OP. The relationship between sarcoidosis and lymphoma. Eur Respir J. 2010. 36:1207–1209.13. Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007. 25:326–333.14. Yao M, Funk GF, Goldstein DP, DeYoung BR, Graham MM. Benign lesions in cancer patients: Case 1. Sarcoidosis after chemoradiation for head and neck cancer. J Clin Oncol. 2005. 23:640–641.15. Kim DS. Sarcoidosis in Korea: report of the Second Nationwide Survey. Sarcoidosis Vasc Diffuse Lung Dis. 2001. 18:176–180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Capecitabine and Cisplatin-induced Cutaneous Hyperpigmentation
- Transient Mutism Related to Capecitabine-Induced Acute Toxic Leukoencephalopathy
- A Case of Coexistent Cutaneous Sarcoidosis in a Patient with Tuberculous Pleurisy
- A Case of Ichthyosiform Sarcoidosis
- Hand-foot Syndrome Due to Capecitabine