Tuberc Respir Dis.
2010 Aug;69(2):81-94.
The Effect of Autophagy to Cell Death in Nutrient-Deprived H460 Cells
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University College of Medicine, Iksan, Korea. yshpul@wonkwang.ac.kr
- 2Department of Pathology, Wonkwang University College of Medicine, Iksan, Korea.
- 3Department of Radiation Oncology, Wonkwang University College of Medicine, Iksan, Korea.
- 4Department of Thoracic Surgery, Wonkwang University College of Medicine, Iksan, Korea.
- 5Department of Nuclear Medicine, Wonkwang University College of Medicine, Iksan, Korea.
- 6Department of Biological Science, Wonkwang University College of Medicine, Iksan, Korea.
Abstract
- BACKGROUND
Autophagy is an important adaptive mechanism in normal development and in response to changing environmental stimuli in cancer. Previous papers have reported that different types of cancer underwent autophagy to obtain amino acids as energy source of dying cells in nutrient-deprived conditions. However, whether or not autophagy in the process of lung cancer causes death or survival is controversial. Therefore in this study, we investigated whether nutrient deprivation induces autophagy in human H460 lung cancer cells.
METHODS
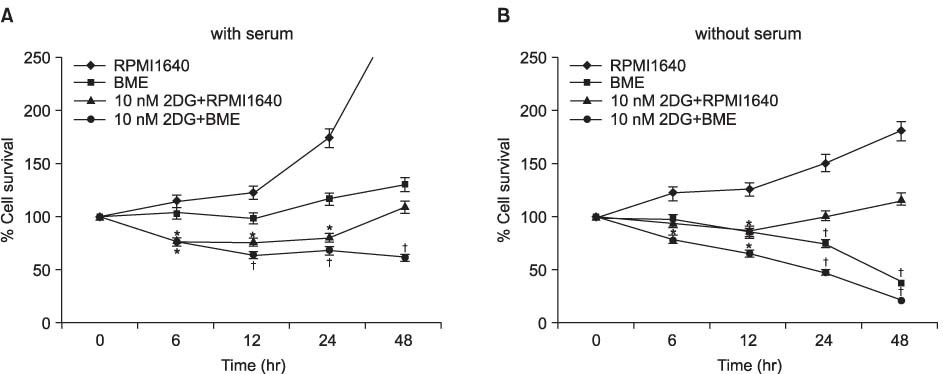
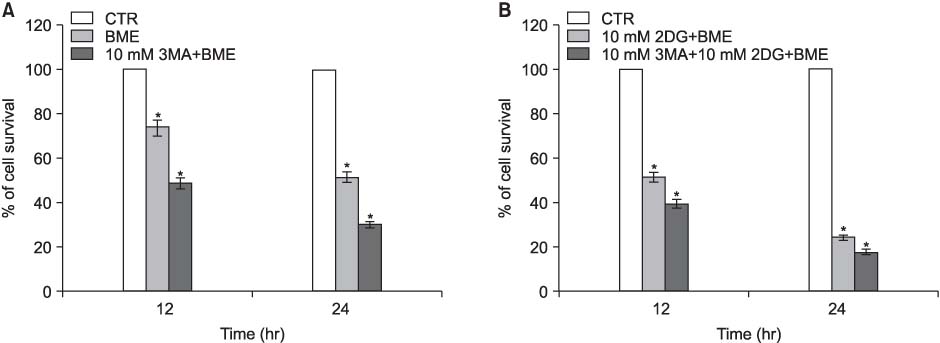
H460, lung cancer cells were incubated in RPMI 1640 medium, and the starved media, which are BME and RPMI media without serum, including 2-deoxyl-D-glucose according to time dependence. To evaluate the viability and find out the mechanism of cell death under nutrient-deprived conditions, the MTT assay and flow cytometry were done and analyzed the apoptotic and autophagic related proteins. It is also measured the development of acidic vascular organelles by acridine orange.
RESULTS
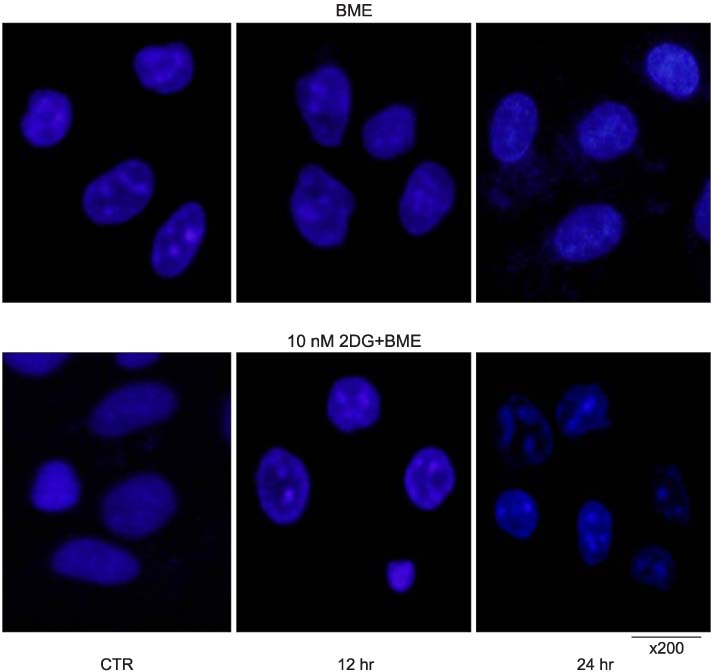
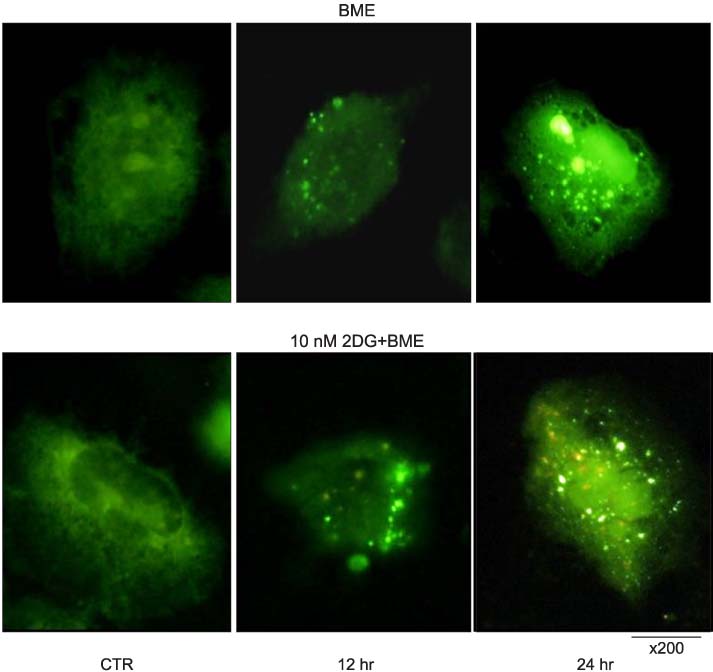
The nutrient-deprived cancer cell is relatively sensitive to cell death rather than normal nutrition. Massive cytoplasmic vacuolization was seen under nutrient-deprived conditions. Autophagic vacuoles were visible at approximately 12 h and as time ran out, vacuoles became larger and denser with the increasing number of vacuoles. In addition, the proportion of acridine orange stain-positive cells increased according to time dependence. Localization of GFP-LC3 in cytoplasm and expression of LC-3II and Beclin 1 were increased according to time dependence on nutrient-deprived cells.
CONCLUSION
Nutrient deprivation induces cell death through autophagy in H460 lung cancer cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Jassem J. Combined chemotherapy and radiation in locally advanced non-small-cell lung cancer. Lancet Oncol. 2001. 2:335–342.2. Bae JM, Won YJ, Jung KW, Suh KA, Ahn DH, Park JG. Annual report of the Central Cancer Registry in Korea-1999: based on registered data from 128 hospitals. Cancer Res Treat. 2001. 33:367–372.3. Ferreira CG, Span SW, Peters GJ, Kruyt FA, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000. 60:7133–7141.4. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol. 2003. 21:2237–2246.5. Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol. 2004. 14:184–193.6. Bowen ID, Mullarkey K, Morgan SM. Programmed cell death during metamorphosis in the blow-fly Calliphora vomitoria. Microsc Res Tech. 1996. 34:202–217.7. Zakeri ZF, Ahuja HS. Apoptotic cell death in the limb and its relationship to pattern formation. Biochem Cell Biol. 1994. 72:603–613.8. Bursch W, Grasl-Kraupp B, Ellinger A, Török L, Kienzl H, Müllauer L, et al. Active cell death: role in hepatocarcinogenesis and subtypes. Biochem Cell Biol. 1994. 72:669–675.9. Dunn WA Jr. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990. 110:1923–1933.10. Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996. 17:1595–1607.11. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003. 63:2103–2108.12. Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001. 61:439–444.13. Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003. 98:378–384.14. Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000. 11:49–61.15. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999. 24:68–72.16. Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988. 240:177–184.17. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997. 3:177–182.18. Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008. 7:809–817.19. Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005. 120:237–248.20. Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002. 302:8–17.21. Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001. 60:1726–1736.22. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002. 418:191–195.23. Kim J, Huang WP, Stromhaug PE, Klionsky DJ. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J Biol Chem. 2002. 277:763–773.24. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000. 19:5720–5728.25. Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 2003. 17:1165–1167.26. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005. 122:927–939.27. Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007. 3:561–568.28. Bursch W, Ellinger A. Autophagy: a basic mechanism and a potential role for neurodegeneration. Folia Neuropathol. 2005. 43:297–310.29. Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005. 1:66–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells
- Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells
- Dual Inhibition of PI3K/Akt/mTOR Pathway and Role of Autophagy in Non-Small Cell Lung Cancer Cells
- Role of Autophagy in the Control of Cell Death and Inflammation
- The role of autophagy in the placenta as a regulator of cell death