Pediatr Gastroenterol Hepatol Nutr.
2014 Mar;17(1):31-36.
Clinical Course of Infliximab Treatment in Korean Pediatric Ulcerative Colitis Patients: A Single Center Experience
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. i101016@skku.edu
- 2Department of Pediatrics, Kyung Hee University Graduate School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Infliximab (IFX) is considered safe and effective for the treatment of ulcerative colitis (UC) in both adults and children. The aim of this study was to evaluate the short- and long-term clinical course of IFX in Korean children with UC.
METHODS
Pediatric patients with UC who had received IFX infusions between November 2007 and May 2013 at Samsung Medical Center were retrospectively investigated. The clinical efficacy of IFX treatment was evaluated at 8 weeks (short term) and 54 weeks (long term) after the initiation of IFX treatment using the Pediatric Ulcerative Colitis Activity Index (PUCAI). The degree of response to IFX treatment was defined as complete response (PUCAI score=0), partial response (decrement of PUCAI score> or =20 points), and non-response (decrement of PUCAI score <20 points). Adverse events associated with IFX treatment were also investigated.
RESULTS
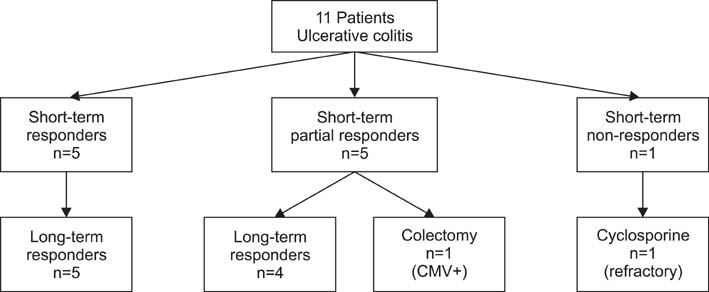
Eleven pediatric patients with moderate to severe UC had received IFX. The remission rate after IFX treatment was 46% (5/11) and 82% (9/11) at 8 weeks and 54 weeks after IFX treatment, respectively. All patients who were steroid-dependent before treatment with IFX achieved remission at 54 weeks and were able to stop treatment with corticosteroids, while all steroid-refractory patients failed to achieve remission at 54 weeks after treatment with IFX.
CONCLUSION
Response to IFX treatment after 8 weeks may predict a favorable long-term response to IFX treatment in Korean pediatric UC patients.
MeSH Terms
Figure
Reference
-
1. Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. 1999; 28:445–458.
Article2. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002; 50:485–489.
Article3. Kader HA, Mascarenhas MR, Piccoli DA, Stouffer NO, Baldassano RN. Experiences with 6-mercaptopurine and azathioprine therapy in pediatric patients with severe ulcerative colitis. J Pediatr Gastroenterol Nutr. 1999; 28:54–58.
Article4. Nielsen OH, Vainer B, Rask-Madsen J. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001; 15:1699–1708.
Article5. Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002; 16:1751–1759.
Article6. Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–1845.
Article7. Treem WR, Cohen J, Davis PM, Justinich CJ, Hyams JS. Cyclosporine for the treatment of fulminant ulcerative colitis in children. Immediate response, long-term results, and impact on surgery. Dis Colon Rectum. 1995; 38:474–479.
Article8. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article9. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007; 132:863–873. quiz 1165-6.
Article10. Eidelwein AP, Cuffari C, Abadom V, Oliva-Hemker M. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis. 2005; 11:213–218.
Article11. Mamula P, Markowitz JE, Brown KA, Hurd LB, Piccoli DA, Baldassano RN. Infliximab as a novel therapy for pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2002; 34:307–311.
Article12. Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, et al. T72 Study Group. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012; 10:391–399.e1.
Article13. Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, et al. European Crohn's and Colitis Organization. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:340–361.14. Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, et al. Pediatric Inflammatory Bowel Disease Collaborative Research Group. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010; 105:1430–1436.
Article15. Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010; 138:2282–2291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Type II Segmental Vitiligo Developed under Infliximab Treatment for Ulcerative Colitis
- Golimumab Therapy in Ulcerative Colitis
- Optic Neuritis after Infliximab Treatment in a Patient with Ulcerative Colitis
- Dual Biological Therapy for Ulcerative Colitis with Intractable Pyoderma Gangrenosum
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis