Korean J Gastroenterol.
2016 Feb;67(2):64-73. 10.4166/kjg.2016.67.2.64.
Golimumab Therapy in Ulcerative Colitis
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. moonone70@hanmail.net
- KMID: 2383543
- DOI: http://doi.org/10.4166/kjg.2016.67.2.64
Abstract
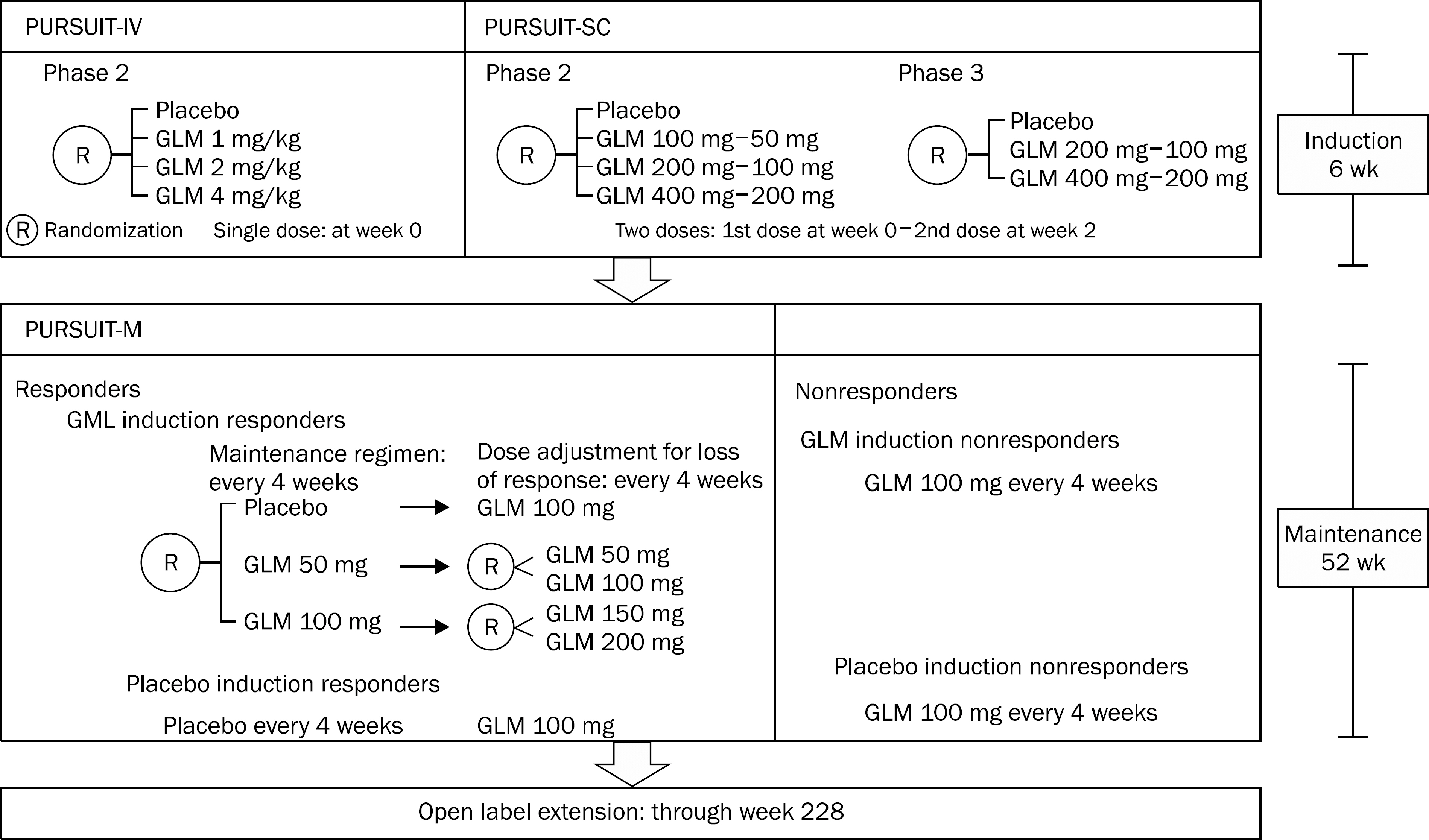
- Ulcerative colitis is a chronic inflammatory condition of the colon, characterized by diffuse mucosal inflammation and blood-mixed diarrhea. The main treatment has been 5-aminosalicylic acid, steroid, thiopurine, and anti-tumor necrosis factor alpha (TNF-alpha) antibodies including infliximab, adalimumab, and golimumab. Golimumab, a new anti-TNF-alpha agent has been recently approved for patients with moderate to severe ulcerative colitis. Its efficacy and safety has been demonstrated in line with infliximab and adalimumab in preclinical and clinical studies. This review will focus on golimumab therapy in ulcerative colitis.
MeSH Terms
-
Antibodies, Monoclonal/blood/*therapeutic use
Antibodies, Monoclonal, Humanized/therapeutic use
Clinical Trials as Topic
Colitis, Ulcerative/*drug therapy
Drug Administration Schedule
Humans
Treatment Outcome
Tumor Necrosis Factor-alpha/immunology
Antibodies, Monoclonal
Antibodies, Monoclonal, Humanized
Tumor Necrosis Factor-alpha
Figure
Reference
-
References
1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725.
Article3. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004; 126:1504–1517.
Article4. Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006; 12(Suppl 1):S3–S9.
Article5. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.6. Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network metaanalysis. Ann Intern Med. 2014; 160:704–711.7. Samaan MA, Bagi P, Vande Casteele N, D'Haens GR, Levesque BG. An update on anti-TNF agents in ulcerative colitis. Gastroenterol Clin North Am. 2014; 43:479–494.
Article8. Campas-Moya C. Golimumab: a novel anti-TNF-alpha human monoclonal antibody for rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Drugs Today (Barc). 2010; 46:13–22.9. Hutas G. Golimumab as the first monthly subcutaneous fully human anti-TNF-alpha antibody in the treatment of inflammatory arthropathies. Immunotherapy. 2010; 2:453–460.10. Shealy DJ, Cai A, Staquet K, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha. MAbs. 2010; 2:428–439.11. Gilardi D, Fiorino G, Allocca M, Bravatà I, Danese S. Golimumab: clinical update on its use for ulcerative colitis. Drugs Today (Barc). 2015; 51:171–184.
Article12. Cesarini M, Fiorino G. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease: an update. Expert Rev Clin Immunol. 2013; 9:301–306.13. Danese S. Nonimmune cells in inflammatory bowel disease: from victim to villain. Trends Immunol. 2008; 29:555–564.
Article14. Paleolog E. Target effector role of vascular endothelium in the inflammatory response: insights from the clinical trial of anti-TNF alpha antibody in rheumatoid arthritis. Mol Pathol. 1997; 50:225–233.
Article15. Martin PL, Oneda S, Treacy G. Effects of an anti-TNF-alpha monoclonal antibody, administered throughout pregnancy and lactation, on the development of the macaque immune system. Am J Reprod Immunol. 2007; 58:138–149.16. Cohen LB, Nanau RM, Delzor F, Neuman MG. Biologic therapies in inflammatory bowel disease. Transl Res. 2014; 163:533–556.
Article17. Ling J, Lyn S, Xu Z, et al. Lack of racial differences in the pharmacokinetics of subcutaneous golimumab in healthy Japanese and Caucasian male subjects. J Clin Pharmacol. 2010; 50:792–802.
Article18. Sandborn WJ, Feagan BG, Marano C, et al. PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95.
Article19. Xu Z, Vu T, Lee H, et al. Population pharmacokinetics of golimumab, an anti-tumor necrosis factoralpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol. 2009; 49:1056–1070.20. Xu Z, Wang Q, Zhuang Y, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010; 50:276–284.
Article21. Xu ZH, Lee H, Vu T, et al. Population pharmacokinetics of golimumab in patients with ankylosing spondylitis: impact of body weight and immunogenicity. Int J Clin Pharmacol Ther. 2010; 48:596–607.
Article22. Zhou H, Jang H, Fleischmann RM, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007; 47:383–396.23. Zhuang Y, Lyn S, Lv Y, et al. Pharmacokinetics and safety of golimumab in healthy Chinese subjects following a single subcutaneous administration in a randomized phase I trial. Clin Drug Investig. 2013; 33:795–800.
Article24. Zhuang Y, Xu Z, Frederick B, et al. Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open-label, randomized study. Clin Ther. 2012; 34:77–90.
Article25. Sandborn WJ, Feagan BG, Marano C, et al. PURSUIT- Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:96–109.e1.
Article26. Smolen JS, Kay J, Doyle MK, et al. GO-AFTER study investigators. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009; 374:210–221.27. Smolen JS, Kay J, Landewé RB, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis. 2012; 71:1671–1679.
Article28. Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with metaanalysis: malignancies with antitumour necrosis factor-a therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014; 39:447–458.29. Rutgeerts P, Feagan BG, Marano CW, et al. PURSUIT-IV study group. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther. 2015; 42:504–514.
Article30. Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a metaanalysis. JAMA. 2012; 308:898–908.31. Xu Z, Marciniak SJ Jr, Frederick B, et al. Pharmacokinetic bridging approach for developing biologics-delivery devices: a case study with a golimumab autoinjector. Clin Ther. 2015; 37:427–438.
Article32. Castro Laria L, Argüelles Arias F, García Sánchez V, et al. Initial experience with golimumab in clinical practice for ulcerative colitis. Rev Esp Enferm Dig. 2016. DOI: doi: 10.17235/reed.2016.4068/2015. [Epub ahead of print].
Article33. Detrez I, Dreesen E, Van Stappen T, et al. Variability in golimumab exposure: a ‘real-life’ observational study in active ulcerative colitis. J Crohns Colitis. 2016. DOI: doi: 10.1093/ecco-jcc/jjv241. [Epub ahead of print].
Article34. Toor K, Druyts E, Jansen JP, Thorlund K. Cost per remission and cost per response with infliximab, adalimumab, and golimumab for the treatment of moderately-to-severely active ulcerative colitis. J Med Econ. 2015; 18:437–446.
Article35. Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network metaanalysis: the efficacy of antitumour necrosis factoralpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2014; 39:660–671.
Article36. Galván-Banqueri M, Vega-Coca MD, Castillo-Muñoz MA, Beltrán Calvo C, Molina López T. Indirect comparison for Anti-TNF drugs in moderate to severe ulcerative colitis. Farm Hosp. 2015; 39:80–91.