Pediatr Gastroenterol Hepatol Nutr.
2012 Jun;15(2):63-73.
Strategy to Overcome Drug Resistance That Develops during Treatment of Chronic Hepatitis B in Children
- Affiliations
-
- 1Department of Pediatrics, Kyungpook National University School of Medicine, Daegu, Korea. bhchoi@knu.ac.kr
Abstract
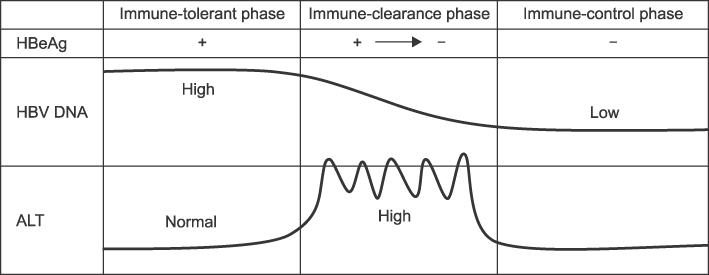
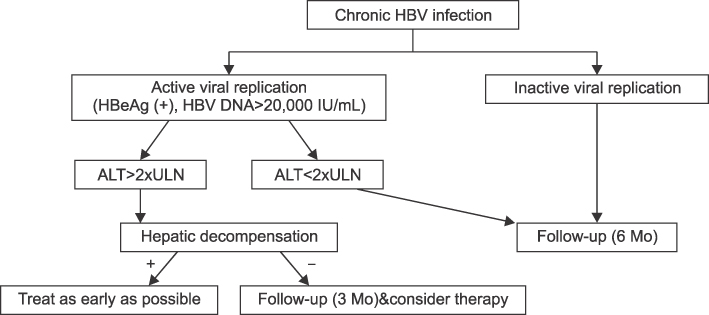
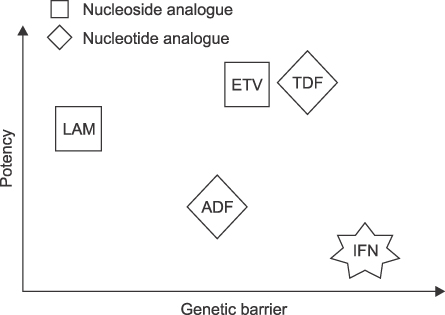
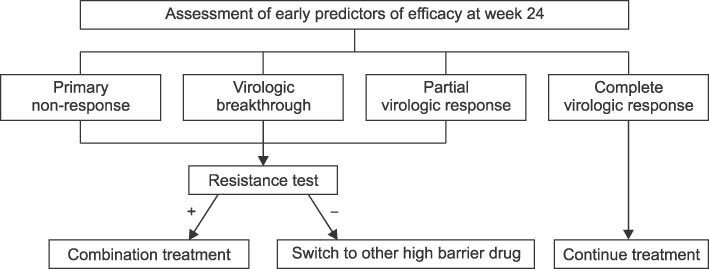
- Development of antiviral resistance to lamivudine is the most important factor for the treatment failure. It is necessary to establish proper guidelines to overcome drug resistance for children with chronic hepatitis B. Primary treatment with lamivudine should be considered if patients are in immune-clearance phase and have persistently elevated ALT levels more than twice the upper limit of normal value. Before initiating the therapy, careful consideration of the patient's status is required to exclude abnormal liver function tests due to other causes. The treatment option should be carefully decided to suppress the viral replication effectively. To obtain good compliance, clinicians should educate patients and their parents. Appropriate monitoring for virologic breakthrough and genotypic resistance is important in deciding to change the treatment plan. Sequential monotherapy should be avoided and a combination of drugs in other categories is recommended. New antiviral agents, such as entecavir and tenofovir, which have high potency and high genetic barrier, are soon expected to be available for use with children.
MeSH Terms
Figure
Reference
-
1. Choe BH. The epidemiology and present status of chronic hepatitis B in Korean children. Korean J Pediatr. 2008. 51:696–703.
Article2. The Korean Association for the Study of the Liver. KASL Guidelines for Treatment of Chronic Hepatitis B. 2011.3. Park HJ, Chu MA, Choe BH. The rate of converting to immune-clearance phase from immune-tolerant phase in children with chronic hepatitis B. 2011. In : The 61st annual fall meeting of the Korea Pediatric Society; 2011; Seoul. 230.4. Shah U, Kelly D, Chang MH, Fujisawa T, Heller S, Gonzalez-Peralta RP, et al. Management of chronic hepatitis B in children. J Pediatr Gastroenterol Nutr. 2009. 48:399–404.
Article5. Jennuvat S, Vithayasai N. Hepatocellular carcinoma in children presents with massive upper gastrointestinal bleeding: a case report. J Med Assoc Thai. 2011. 94:Suppl 3. S222–S225.6. Hsu HC, Wu MZ, Chang MH, Su IJ, Chen DS. Childhood hepatocellular carcinoma develops exclusively in hepatitis B surface antigen carriers in three decades in Taiwan. Report of 51 cases strongly associated with rapid development of liver cirrhosis. J Hepatol. 1987. 5:260–267.
Article7. Lee HC, Kim YJ, Seo JJ, Moon HN, Ghim TT. Primary hepatic tumors in children: Clinical experience in a single institution. Korean J Pediatr Hematol Oncol. 2000. 7:269–277.8. Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, et al. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology. 2006. 43:556–562.
Article9. Kim JO, Choe BH. Long-term lamivudine treatment in children and adolescence with chronic hepatitis B: predictors of therapeutic response and lamivudine resistance. 2008. 2008; Seoul. Asian Pacific Association for the Study of the Liver (APASL);A220.10. Torre D, Tambini R. Interferon-alpha therapy for chronic hepatitis B in children: a meta-analysis. Clin Infect Dis. 1996. 23:131–137.11. Jonas MM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002. 346:1706–1713.
Article12. Chang MH, Chen PJ, Chen JY, Lai MY, Hsu HC, Lian DC, et al. Hepatitis B virus integration in hepatitis B virus-related hepatocellular carcinoma in childhood. Hepatology. 1991. 13:316–320.
Article13. Choe BH. Consulting about chronic hepatitis B: Focusing on common errors of internet website in Korea. Korean J Pediatr Gastroenterol Nutr. 2008. 11:1–11.
Article14. Lai CL, Lok AS, Lin HJ, Wu PC, Yeoh EK, Yeung CY. Placebo-controlled trial of recombinant alpha 2-interferon in Chinese HBsAg-carrier children. Lancet. 1987. 2:877–880.15. Hom X, Little NR, Gardner SD, Jonas MM. Predictors of virologic response to Lamivudine treatment in children with chronic hepatitis B infection. Pediatr Infect Dis J. 2004. 23:441–445.
Article16. Kim JS, Jeong JY. Predictors of response to lamivudine treatment in children with chronic hepatitis B. Chonnam Med J. 2008. 44:37–42.
Article17. Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998. 114:988–995.
Article18. Choe BH, Kim JM, Lee JH, Jang YC, Jang CH, Oh KW. Lamivudine treatment in children with chronic hepatitis B: Long-term therapeutic response, optimal duration and resistance. 2006. In : The 39th Annual Meeting of European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN); 2006; Dresden, Germany. 47.19. Jonas MM, Block JM, Haber BA, Karpen SJ, London WT, Murray KF, et al. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology. 2010. 52:2192–2205.
Article20. Choe BH, Lee JH, Jang YC, Jang CH, Oh KW, Kwon S, et al. Long-term therapeutic efficacy of lamivudine compared with interferon-alpha in children with chronic hepatitis B: the younger the better. J Pediatr Gastroenterol Nutr. 2007. 44:92–98.
Article21. Choe BH. The management and treatment of chronic hepatitis B in Korean children. Korean J Pediatr. 2007. 50:823–834.
Article22. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009. 50:661–662.
Article23. Leung N. Recent data on treatment of chronic hepatitis B with nucleos(t)ide analogues. Hepatol Int. 2008. 2:163–178.
Article24. Kurbegov AC, Sokol RJ. Hepatitis B therapy in children. Expert Rev Gastroenterol Hepatol. 2009. 3:39–49.
Article25. National Health Insurance Corporation. The Criterias for the Medical Care Benefits. 2010. Available from: http://www.nhic.or.kr.26. Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008. 49:634–651.
Article27. Hong SP, Han KH, Ahn SH, Paik YH, Moon BS, Chon CY, et al. Long-term Efficacy and Durability of Lamivudine Therapy in Patients with Chronic Hepatitis B. Korean J Hepatol. 2001. 7:423–431.28. Koh H, Baek SY, Chung KS. Lamivudine therapy for korean children with chronic hepatitis B. Yonsei Med J. 2007. 48:927–933.
Article29. Lee EH, Jang JY, Kim KM. Efficacy of lamivudine therapy for chronic hepatitis B in children. Korean J Pediatr Gastroenterol Nutr. 2008. 11:130–136.
Article30. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005. 352:2682–2695.
Article31. Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005. 365:123–129.
Article32. Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005. 142:240–250.
Article33. Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004. 351:1206–1217.
Article34. Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000. 46:562–568.
Article35. Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008. 48:728–735.
Article36. Dikici B, Ozgenc F, Kalayci AG, Targan S, Ozkan T, Selimoglu A, et al. Current therapeutic approaches in childhood chronic hepatitis B infection: a multicenter study. J Gastroenterol Hepatol. 2004. 19:127–133.
Article37. Sokucu S, Gokce S, Suoglu OD, Emiroglu H, Cevikbas U. Comparison of interferon monotherapy with interferon-lamivudine combination treatment in children with chronic hepatitis B. Indian J Gastroenterol. 2006. 25:136–139.38. Carey I, Harrison PM. Monotherapy versus combination therapy for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2009. 18:1655–1666.
Article39. Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010. 51:415–421.
Article40. Kim JM, Choi BH, Chu MA, Cho SM, Choe BH. Clinical experience with long-term lamivudine therapy to determine the adequate duration of treatment in childrenand adolescents with HBeAg-negative chronic hepatitis B. Korean J Pediatr Gastroenterol Nutr. 2009. 12:23–29.
Article41. Yim HJ. Management of antiviral-resistant chronic hepatitis B virus infection. Korean J Gastroenterol. 2008. 51:346–359.42. Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res. 2004. 64:1–15.
Article43. Chu MA, Cho SM, Choe BH, Cho MH, Kwon SH, Lee WK. Virologic responses to add-on adefovir dipivoxil treatment versus entecavir monotherapy in children with lamivudine-resistant chronic hepatitis B. J Pediatr Gastroenterol Nutr. 2012. in press.
Article44. Choe BH. Hepatitis B virus: pathogenesis, molecular diagnosis, and clinical significance of mutation. Korean J Pediatr Gastroenterol Nutr. 2007. 10:51–65.45. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007. 357:2576–2588.
Article46. Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001. 34:785–791.
Article47. Fukai K, Imazeki F, Kurihara T, Mikata R, Yokosuka O. Association between lamivudine sensitivity and the number of substitutions in the reverse transcriptase region of the hepatitis B virus polymerase. J Viral Hepat. 2007. 14:661–666.
Article48. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008. 6:1315–1341. quiz 286.
Article49. Keeffe EB, Zeuzem S, Koff RS, Dieterich DT, Esteban-Mur R, Gane EJ, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007. 5:890–897.
Article50. Reijnders JG, Deterding K, Petersen J, Zoulim F, Santantonio T, Buti M, et al. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J Hepatol. 2010. 52:493–500.
Article51. Ahn SH, Lee HJ, Tak WY, Um SH, Kim DY. Prospective randomized trial of switching to entecavir in chronic hepatitis B patients with suboptimal virologic response to lamivudine: Interim analysis at 48 Weeks. Hepatology Int. 2010. 4:53A.52. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003. 348:800–807.
Article53. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005. 352:2673–2681.
Article54. Hwang SK, Park SM, Choe BH, Kim JM, Kim JO, Kim YM, et al. Therapeutic efficacy of adefovir dipivoxil in Korean children and adolescents with chronic hepatitis B who have developed lamivudine resistance. Korean J Pediatr Gastroenterol Nutr. 2008. 11:143–149.
Article55. Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006. 55:1488–1495.
Article56. Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006. 43:1385–1391.
Article57. Gaia S, Barbon V, Smedile A, Olivero A, Carenzi S, Lagget M, et al. Lamivudine-resistant chronic hepatitis B: an observational study on adefovir in monotherapy or in combination with lamivudine. J Hepatol. 2008. 48:540–547.
Article58. Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, et al. Rescue therapy for lamivudine-resistant chronic hepatitis B: comparison between entecavir 1.0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. J Gastroenterol Hepatol. 2010. 25:1374–1380.
Article59. Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004. 126:81–90.
Article60. Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004. 126:91–101.61. Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007. 45:307–313.
Article62. Shin SR, Jung HY, Gwak GY, Choi MS, Lee JH, Paik SW, et al. The DNA levels on initiation of adefovir as the important factor to get the more initial virological response in patients with lamivudine resistant hepatitis B. Hepatology. 2008. 48:738A.63. Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008. 48:99–108.
Article64. Osborn M. Safety and efficacy of entecavir for the treatment of chronic hepatitis B. Infect Drug Resist. 2011. 4:55–64.
Article65. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006. 354:1001–1010.
Article66. Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007. 133:1437–1444.
Article67. Baldick CJ, Eggers BJ, Fang J, Levine SM, Pokornowski KA, Rose RE, et al. Hepatitis B virus quasispecies susceptibility to entecavir confirms the relationship between genotypic resistance and patient virologic response. J Hepatol. 2008. 48:895–902.
Article68. Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004. 48:3498–3507.
Article69. Villet S, Ollivet A, Pichoud C, Barraud L, Villeneuve JP, Trepo C, et al. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007. 46:531–538.
Article70. Chung GE, Kim W, Lee KL, Hwang SY, Lee JH, Kim HY, et al. Add-on adefovir is superior to a switch to entecavir as rescue therapy for Lamivudine-resistant chronic hepatitis B. Dig Dis Sci. 2011. 56:2130–2136.
Article71. Ryu HJ, Lee JM, Ahn SH, Kim do Y, Lee MH, Han KH, et al. Efficacy of adefovir add-on lamivudine rescue therapy compared with switching to entecavir monotherapy in patients with lamivudine-resistant chronic hepatitis B. J Med Virol. 2010. 82:1835–1842.
Article72. Yim HJ, Yoon E, Seo YS, Kim CW, Lee CD, Park SH, et al. Adding adefovir compared with switching to entecavir in patients with lamivudine-resistant chronic hepatitis B (ACE study) - A multicenter prospective randomized study: 2 year final results. Hepatology. 2011. 54:Suppl 1. 1060–1061A.73. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003. 125:1714–1722.
Article74. Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, et al. Lamivudine Clinical Investigation Group. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology. 1998. 27:1670–1677.
Article75. Yoe S, Na SY, Yang HR, Chang JY, Ko JS, Kim BS, et al. Incidence and risk factors of the YMDD mutation during lamivudine therapy in children with chronic hepatitis B. 2009. 2009; Seoul. Asian Pan-Pacific Society of Pediatric Gastroenterology, Hepatology and Nutrition (APPSPGHAN);106.76. Sokal EM, Kelly DA, Mizerski J, Badia IB, Areias JA, Schwarz KB, et al. Long-term lamivudine therapy for children with HBeAg-positive chronic hepatitis B. Hepatology. 2006. 43:225–232.
Article77. Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, et al. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003. 37:1172–1179.
Article78. Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003. 23:47–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring and Treatment Strategy for Chronic Hepatitis B and Antiviral Resistant Hepatitis B
- Lamivudine and adefovir combination therapy in lamivudine-resistant HBeAg-positive chronic hepatitis B patients
- Options for the management of antiviral resistance during hepatitis B therapy: reflections on battles over a decade
- Resistance to Adefovir in Patients with Chronic Hepatitis B
- Is tenofovir monotherapy a sufficient defense line against multi-drug resistant hepatitis B virus?