Korean J Urol.
2010 Dec;51(12):870-878.
Dimethoxycurcumin, a Structural Analogue of Curcumin, Induces Apoptosis in Human Renal Carcinoma Caki Cells Through the Production of Reactive Oxygen Species, the Release of Cytochrome c, and the Activation of Caspase-3
- Affiliations
-
- 1Department of Urology, Wonkwang University School of Medicine, Iksan, Korea.
- 2Department of Urology, Chonnam National University School of Medicine, Gwangju, Korea.
- 3Department of Microbiology and Immunology, Wonkwang University School of Medicine, Iksan, Korea. hopae@wonkwang.ac.kr
Abstract
- PURPOSE
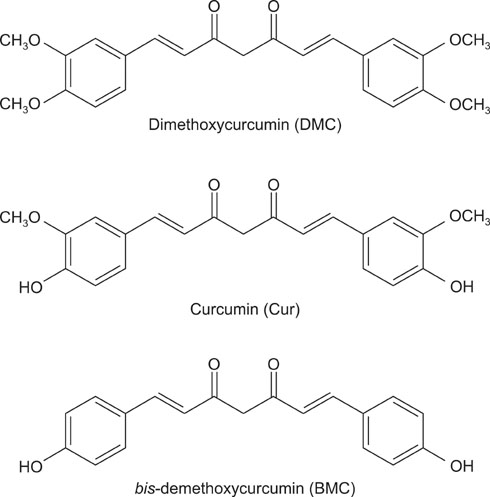
Curcumin (Cur) has been reported to induce apoptosis in human renal carcinoma Caki cells. Dimethoxycurcumin (DMC), one of several synthetic Cur analogues, has been reported to have increased metabolic stability over Cur. We determined whether DMC, like Cur, induces apoptosis in Caki cells and also compared the apoptosis-inducing activity of DMC with that of Cur.
MATERIALS AND METHODS
Caki cells were treated with DMC possessing four methoxy groups, Cur possessing two methoxy groups, or bis-demethoxycurcumin (BMC), which lacks a methoxy group. Cell viability was measured by using a methyltetrazolium assay. Flow cytometry and the caspase-3 activity assay were used to detect apoptosis. The release of cytochrome-c (Cyt c) was detected by Western blot analysis. The production of reactive oxygen species (ROS) was measured by flow cytometry.
RESULTS
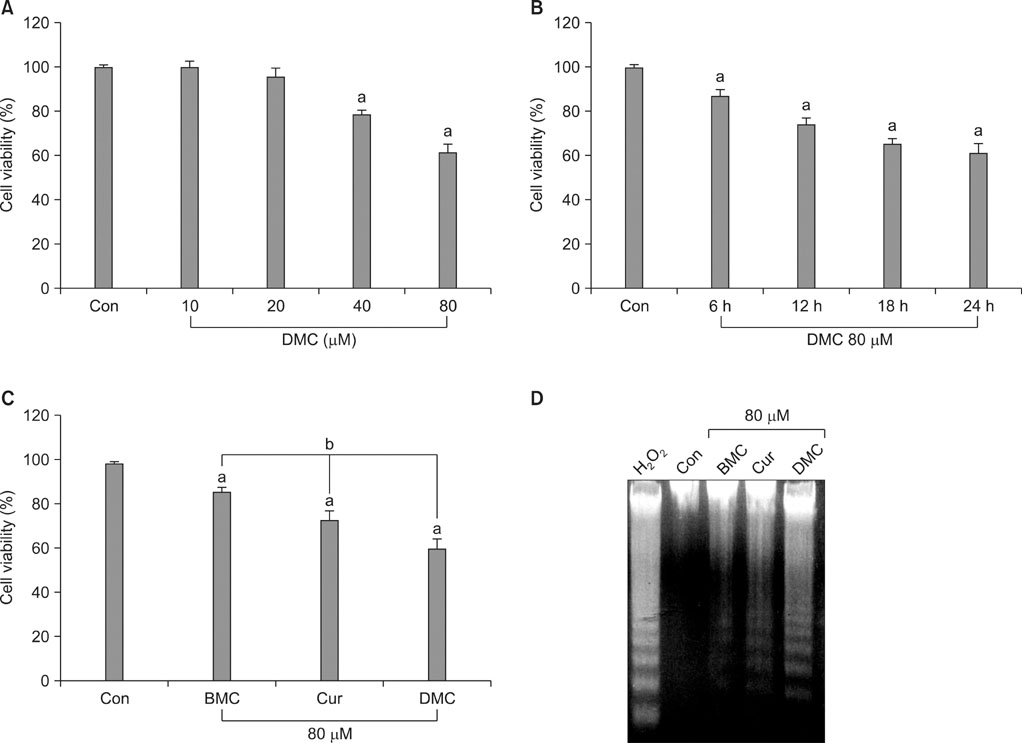
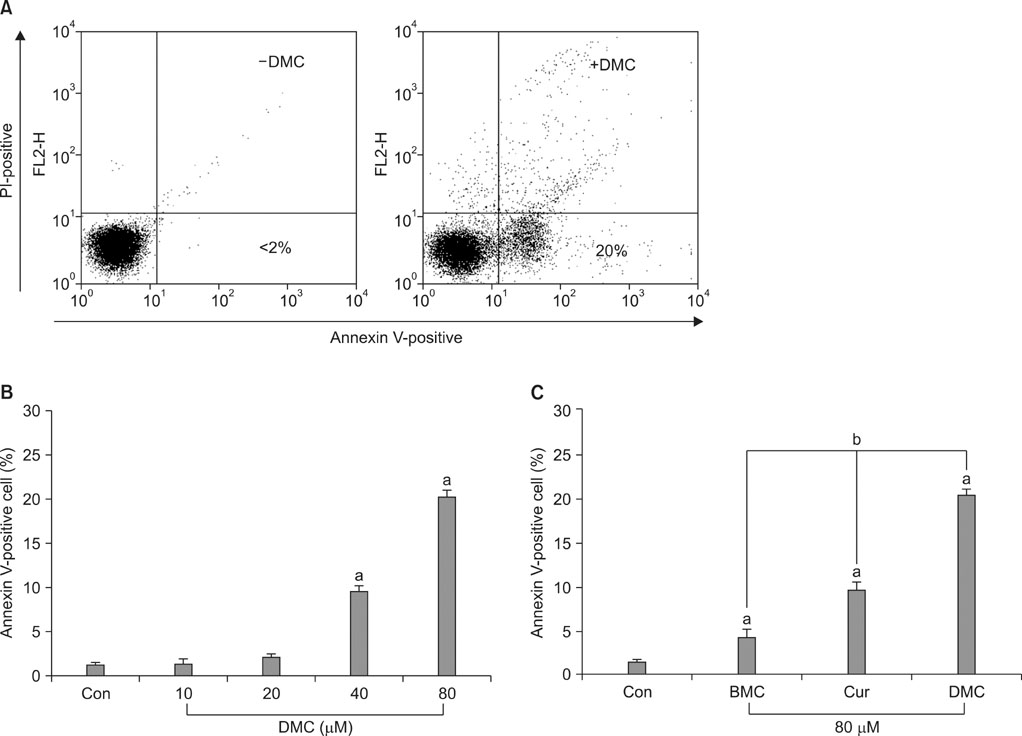
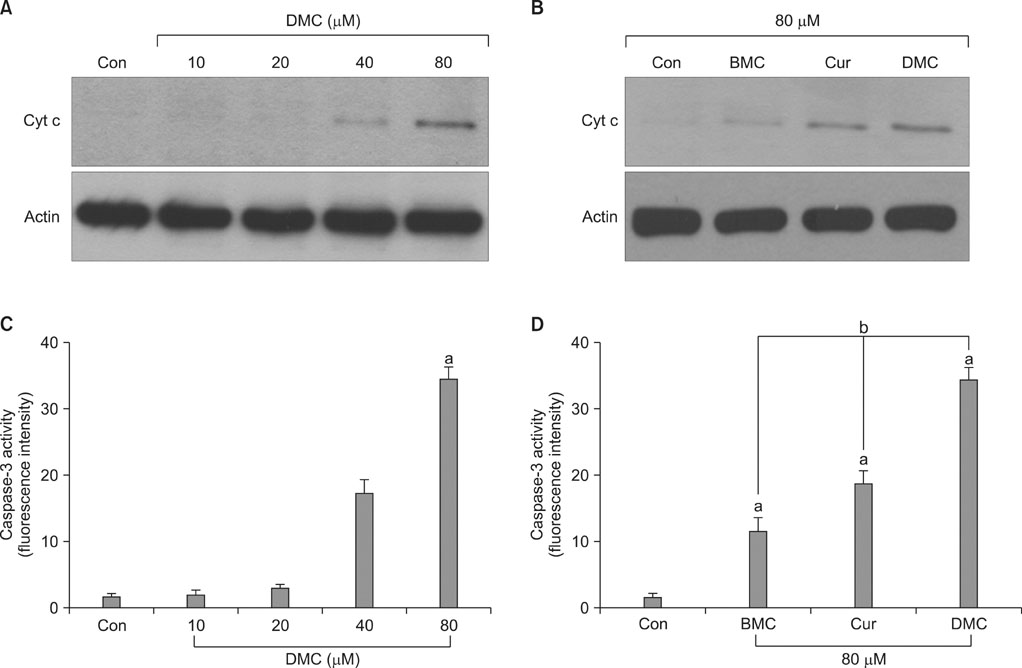
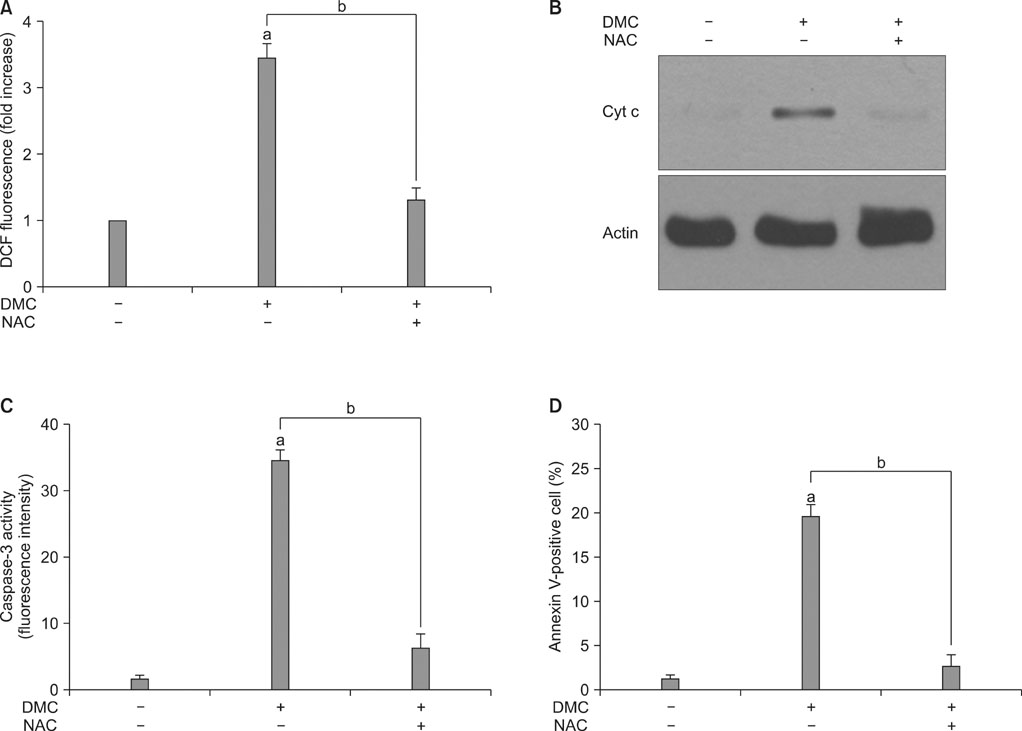
DMC, Cur, and BMC reduced cell viability and induced apoptosis, but the potency varied; DMC was the most potent compound, followed by Cur and BMC. ROS production, Cyt c release, and caspase-3 activity were increased, again in the order DMC>Cur>BMC. N-Acetylcysteine, a potent antioxidant, inhibited ROS production, Cyt c release, caspase-3 activation, and apoptosis induction in DMC-treated cells.
CONCLUSIONS
These results indicate that DMC, like the original form of Cur, may induce apoptosis in human renal carcinoma Caki cells through the production of ROS, the release of mitochondrial Cyt c, and the subsequent activation of caspase-3. In addition, DMC is more potent than Cur in the ability to induce apoptosis.
MeSH Terms
-
Acetylcysteine
Antineoplastic Agents
Apoptosis
Blotting, Western
Carcinoma, Renal Cell
Caspase 3
Cell Survival
Chlorobenzenes
Curcumin
Cytochromes
Cytochromes c
Flow Cytometry
Humans
Reactive Oxygen Species
Acetylcysteine
Antineoplastic Agents
Caspase 3
Chlorobenzenes
Curcumin
Cytochromes
Cytochromes c
Reactive Oxygen Species
Figure
Reference
-
1. Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010. 17:190–197.2. Nagabhushan M, Bhide SV. Curcumin as an inhibitor of cancer. J Am Coll Nutr. 1992. 11:192–198.3. Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M. Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[α]anthracene. Carcinogenesis. 1998. 19:1039–1043.4. Tang H, Murphy CJ, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, et al. Curcumin polymers as anticancer conjugates. Biomaterials. 2010. 31:7139–7149.5. Mehta K, Pantazis P, McQueen T, Aggarwal BB. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs. 1997. 8:470–481.6. Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010. 17:R39–R52.7. Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008. 76:1340–1351.8. Chen Q, Wang Y, Xu K, Lu G, Ying Z, Wu L, et al. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol Rep. 2010. 23:397–403.9. Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 2008. 45:1403–1412.10. Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006. 26:4379–4389.11. Mishra S, Kapoor N, Mubarak Ali A, Pardhasaradhi BV, Kumari AL, Khar A, et al. Differential apoptotic and redox regulatory activities of curcumin and its derivatives. Free Radic Biol Med. 2005. 38:1353–1360.12. Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999. 456:311–314.13. Hadi SM, Asad SF, Singh S, Ahmad A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life. 2000. 50:167–171.14. Hsu YC, Weng HC, Lin S, Chien YW. Curcuminoids-cellular uptake by human primary colon cancer cells as quantitated by a sensitive HPLC assay and its relation with the inhibition of proliferation and apoptosis. J Agric Food Chem. 2007. 55:8213–8222.15. Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001. 61:1058–1064.16. Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002. 11:105–111.17. Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007. 13:1269–1277.18. Basu B, Eisen T. Perspectives in drug development for metastatic renal cell cancer. Target Oncol. 2010. 5:139–156.19. Varella L, Rini BI. Emerging drugs for renal cell carcinoma. Expert Opin Emerg Drugs. 2010. 15:343–353.20. Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003. 24:1199–1208.21. Kim DG, Kwon TK, Park JW, Lee KS. Curcumin induces apoptosis and inhibits metalloproteinase activity in renal cancer cell Line. Korean J Urol. 2002. 43:423–430.22. Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp Mol Med. 2006. 38:393–400.23. Pae HO, Jeong SO, Kim HS, Kim SH, Song YS, Kim SK, et al. Dimethoxycurcumin, a synthetic curcumin analogue with higher metabolic stability, inhibits NO production, inducible NO synthase expression and NF-kappaB activation in RAW264.7 macrophages activated with LPS. Mol Nutr Food Res. 2008. 52:1082–1091.24. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010. 48:749–762.25. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007. 595:471–480.26. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007. 4:807–818.27. Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010. 44:479–496.28. Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006. 13:1423–1433.29. Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009. 11:495–510.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin Induces Apoptosis and Inhibits Metalloproteinase Activity in Renal Cancer Cell Line
- Domperidone Induces Apoptosis through Suppression of STAT3 Signaling in Human Renal Cancer Caki-2 Cells
- Effect of curcumin and resveratrol on the cell cycle regulation, apoptosis and inhibition of metastasis related proteins in HN-4 cells
- Paraquat Induces Apoptosis through Cytochrome C Release and ERK Activation
- Arsenic Trioxide Induces A poptosis in Human Colorectal Adenocarcinoma HT-29 Cells Through ROS